In this blog, I am going to cover the common IAL Chemistry Unit-3 practical written examination common questions and their answers.
To simplify things I have grouped the common questions into different topics. I have analyzed over 10 years of IAL Chemistry Unit-3 question papers. I am providing the questions which appear most frequently.
This blog will definitely help you get a lot of marks in the bag during your IAL Chemistry Unit-3 exam. Hope you find this blog useful, enjoy!
Topic 1: Detecting HCO3– / CO3-2 ions
When carbonate ions (CO3-2) ions or Hydrogencarbonate (HCO3–) ions react with acid, they release CO2 gas, so if any of these two ions are present in a solution or solid, in addition to acid you will see fizzing/effervescence/ bubbling. In general, case write that the ion is CO3-2
Common Questions
If J contained a carbonate anion, what would be the observation on the addition of dilute Hydrochloric acid?
Answer: Effervescence, fizzing, bubbling
Give the name or formula of another anion that would produce the same observation with dilute hydrochloric acid as the carbonate ion.
Answer: HCO3– (Hydrogen Carbonate ion)
When dilute nitric acid was added to a sample of solid x, no reaction occurred. Suggest the name or formula of an anion that could not be present in x.
Answer: CO3-2
| Test for CO3-2 or HCO3-2 ions | Result | Interpretation |
|---|---|---|
| Add dilute Hydrochloric acid to the solid X , then bubble the gas given off into limewater (Limewater is Calcium Hydroxide solution) | Effervescence, The limewater turns milky | Anion X is CO3-2 or HCO3-2 |
Revise IAL Chemistry unit 3 within 45 minutes & get above 90%
- No BS, to the point, quick, and easy to revise.
- Actual common questions with answers from the question paper. Get easy marks in the bag during your exam.
- Questions are divided into different topics.
- A brief discussion on each topic to get your concepts clear.
- Available in kindle ebook and paperback formats on Amazon
Topic 2: Testing for H+ ions/whether solution is acidic:
As we have seen from the above, carbonate and hydrogen carbonate ion reacts with acid/H+ ions to form carbon dioxide gas, which causes fizzing/bubbling/effervescence. So if we have an acidic solution containing H+ ion, in addition of carbonate ion there would be fizzing. The carbonate ion is added in the form of Na2CO3 solution. This solution can also be used to remove any excess H+ ions, as we know carbonate ion uses up H+ ions.
Common Questions
Suggest a reagent that is used to confirm the presence of an acid group in either of the two compounds above and the positive observation that would be made.
Answer:
Reagent: Na2CO3
Observation: fizzing, effervescence, bubbling
Suggest why, in step 6, the crude iodoethane is washed with dilute sodium carbonate solution
Answer: To neutralize acid
The 1- is then washed with dilute sodium carbonate solution
What is the purpose of this step?
Answer: To neutralize acid
Explain why the 1- is shaken with sodium carbonate solution in step 5
Answer: To neutralize acid
Suggest why each sample was added to sodium hydrogen carbonate (NaHCO3) solution. Explain your answer.
Answer: to neutralize the acid
State the purpose of the following in this procedure:
Washing the product with dilute sodium carbonate solution (step 6)
Answer: to neutralize acid
The product is washed with concentrated hydrochloric acid in step 7 to some unreacted butan-1-ol in step 8, why is this product washed with sodium hydrogen carbonate solution and what causes the buildup of gas?
Answer: To neutralize acid, formation of CO2 gas
Explain the purpose of the sodium hydrogen carbonate solution
Answer: to neutralize acid
Washing the product with dilute sodium carbonate solution
Answer: To neutralize the acid
Topic 3: Infrared Spectroscopy
Some questions ask about the infrared spectroscopy of different compounds, the infrared spectroscopy shows absorptions peaks for the bonds –OH, C=O, etc. Suppose in alcohol and a carboxylic acid, both of them have –OH peaks, but the alcohol does not have any peaks for the C=O bond. So this is how they are differentiated, you may be given different compounds, analyze and tell the difference in terms of what peaks are present or absent. Examples of common questions with answers are below.
Common Questions
Identify one way in which the infrared spectrum of propanedioic acid would be different from that of the infrared spectrum of propane-1,3-diol. Wavenumber not required.
Answer: C=O peak present in propanedioic acid but absent in propane-1,3 diol.
One of the isomers of the alcohol Q is an ether. Ether contains two alkyl groups linked by an oxygen atom and can be represented as R-O-R.
Explain how the information in an infrared spectrum would be used to decide whether the spectrum is produced by an alcohol or an ether. Wavenumber data are not required.
Answer: Alcohol has peak for the –OH but ether does not.
How could infrared spectroscopy be used to show that two isomers of Z both have an –OH group? You are not required to give wavenumber values
Answer: both of them will have an absorption peak for the -OH
Topic 4: Why Yield is not 100%
Give one reason why the actual yield is lower than the maximum possible yield
Answer: Handling/transfer losses/ competing reactions
Suggest one reason, other than volatility of the reagent or products, why the preparation does not produce 100% yield.
Answer: Handling/transfer losses/ competing reactions
Topic 5: Reaction of alcohol with sodium
When alcohol reacts with sodium, hydrogen gas is produced and the sodium gets smaller. Please refer to the common questions below.
Reaction equation between Sodium and Ethanol (alcohol):
2CH3CH2OH + 2Na → 2CH3CH2ONa + H2
Common Questions
Under suitable conditions, each of the compounds react slowly with a small piece of sodium to form sodium salt and one other product. Give two observations you would make when this reaction occurs.
Answer:
Effervescence/fizzing/bubbles, sodium dissolves
State two observations you would expect to make when a small piece of sodium is added to C.
Answer:
Effervescence/fizzing/bubbles, sodium dissolves
Reminder: you can get the extended version of these common questions and answers Edexcel IAL Chemistry Unit 3 Common Questions and Answers: Study and Revision Guide from Amazon in kindle or paperback formats.
Revise IAL Chemistry unit 3 within 45 minutes & get above 90%
- No BS, to the point, quick, and easy to revise.
- Actual common questions with answers from the question paper. Get easy marks in the bag during your exam.
- Questions are divided into different topics.
- A brief discussion on each topic to get your concepts clear.
- Available in kindle ebook and paperback formats on Amazon
Topic 6: Reactions which needs to be heated to occur
Examples:
NH4Cl ➝ NH3 + HCl
CaCO3 ➝ CaO + CO2
CuCO3 ➝ CuO + CO2
Or any other reactions in which heat is required, it is not possible to measure the enthalpy change, because heat is being given, it is not possible to determine what amount of heat is needed for the reaction to occur. The same case will occur for other reactions which need heat to occur.
Common Questions
Suggest why it is not possible to determine directly the enthalpy change for the thermal decomposition of copper(II)carbonate
Answer: Difficult to measure heat absorbed when heating any substance
The equation for the thermal decomposition of ammonium chloride is shown below.
NH4Cl(s) ➝ NH3(g) + HCl(g)
Suggest why the enthalpy change for this reaction, ΔHreaction, is difficult to determine directly by experiment.
Answer: Difficult to measure heat absorbed when heating any substance
Topic 7: Mass Spectrometer
The relative molecular mass of a compound or molecule can be found out from the molecular ion peak. It is the peak with the maximum m:z ratio (mass :charge ratio) . Also if two molecules are isomers of each other they will have the same molecular ion peak, as they both have the same mass.
Common Questions
How the relative molecular mass of a compound be found from its mass spectrum
Answer: from the peak with the maximum mass: charge (m:z) ratio
Give one piece of evidence from their mass spectra which would show that two compounds could be isomers.
Answer: Molecular ion peaks have the same m:z ratio
Topic 8: Exothermic Reaction
Whenever a reaction is exothermic, the reagents must be added slowly, because the reaction might become vigorous. So whenever a question asks why a reagent being added slowly, the answer will simply be exothermic or just that the reaction is vigorous. If not then state other reasons, please see the common questions.
Common Questions
The reaction is exothermic. Other than the risk of explosion why it is important to cool the flask in a beaker with ice and water in step 2.
Answer: solvent may ignite
Step 3: A suitable quantity of powdered iodine is added in small portions down the condenser at a rate which just maintains gentle boiling. The reaction should be allowed to subside after each addition
What does the manner in which the iodine is added in step 3 suggests about the nature of the reaction?
Answer: Exothermic
Explain why the acid must be added slowly with cooling in step 2
Answer: Exothermic reaction
Suggest why the mixture was cooled before the water was added.
Answer: exothermic reaction
Give one reason why the iodine was added over a period of time and in small amounts
Answer: Exothermic reaction
Explain why water baths were used in both step 6 and step 8 rather than heating the flasks directly with a Bunsen flame.
Answer: The reaction is vigorous
Topic 9: Indicators
It is very important to remember the colors of some indicators in an acidic, neutral and basic state. Here below, I am noting down the colors of Methyl Orange and Phenolphthalein.
| Indicator Name | Alkaline (pH > 7) | Neutral (pH = 7) | Acidic (pH<7) |
|---|---|---|---|
| Methyl Orange | Yellow | Orange | Red |
| Phenolphthalein | Pink | Colorless | Colorless |
Common Questions
A student suggested using Universal Indicator for the titration. Why would this indicator be unsuitable?
Answer: Because the universal indicator does not give a sharp/prominent color change.

Name the indicator that is used in thiosulfate/iodine titrations
Answer: starch
At what stage in the titration should this indicator be added?
Answer: As the color of the iodine starts to fade.
Revise IAL Chemistry unit 3 within 45 minutes & get above 90%
- No BS, to the point, quick, and easy to revise.
- Actual common questions with answers from the question paper. Get easy marks in the bag during your exam.
- Questions are divided into different topics.
- A brief discussion on each topic to get your concepts clear.
- Available in kindle ebook and paperback formats on Amazon
Topic 10: Drying agent:
The drying agent simply removes water. One of the most used drying agents is anhydrous calcium chloride, the following common questions also cover more drying agents. Please have a look at those.
Common Questions
What is the purpose of adding sodium sulfate in step 5?
Answer: drying agent
Suggest a suitable drying agent to use in step 4?
Answer: Anhydrous Calcium Chloride, Anhydrous magnesium sulfate, Anhydrous sodium sulfate
State why calcium chloride used in step 8 must be anhydrous?
Answer: Hydrated salt will not remove water
What is the purpose of calcium chloride in step 6?
Answer: drying agent
Why adding anhydrous calcium chloride to organic layer?
Answer: drying agent
Explain the difference between a ‘dehydrating agent’, such as the phosphoric acid used in Step 1, and a ‘drying agent’, such as the anhydrous calcium chloride used in Step 4.
Answer: removes water in a (chemical) reaction, removes water mixed with other materials
Reminder: you can get the extended version of these common questions and answers Edexcel IAL Chemistry Unit 3 Common Questions and Answers: Study and Revision Guide from Amazon in kindle or paperback formats.
Topic 11: Making Halogenoalkane from alcohol:
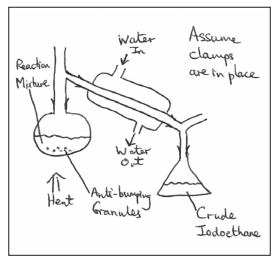
• Halogenoalkane can be prepared in the labrotary using butan-1-ol sodium bromide and concentrated sulfuric acid.
• Firstly NaBr reacts with H2SO4 to produce HBr, which then reacts with the alcohol to produce the halogenoalkanes.
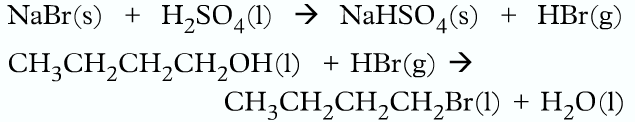
- The flask is then put into cold water as the reaction is exothermic
- The dropping funnel is removed and the apparatus is distilled for 30 minutes
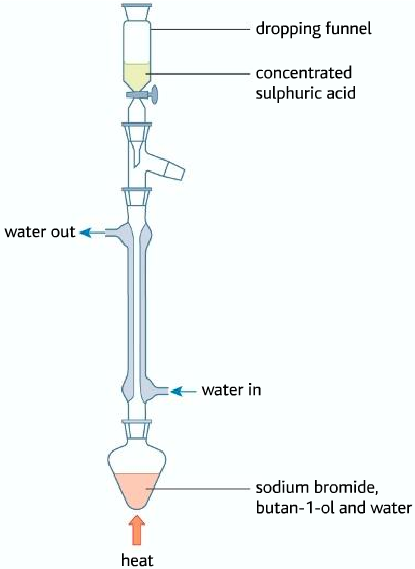
- Then it is distilled.
• Halogenoalkane distills in the organic layer
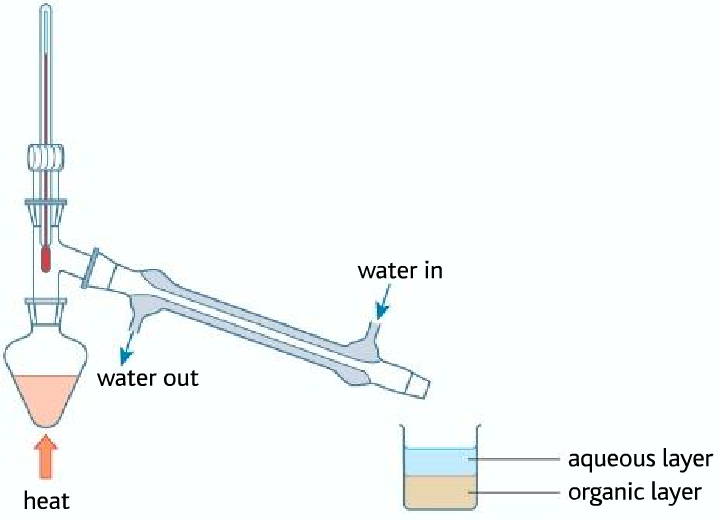
• Problem of using this process to make iodoalkane, the problem is that the sulfuric acid oxidizes iodide ions to iodine and produces hardly any hydrogen iodide
As it can be seen that the halogenoalkane is in the organic layer, containing various impurities. The impure halogenoalkane is known as crude (impure, unrefined). So therefore it must be purified using various methods.
Purifying the bromobutane
Impurities in the bromobutane include:
• hydrogen bromide (although most of that will dissolve in the water if you are collecting the bromobutane underwater);
• bromine – from the oxidation of bromide ions by the concentrated sulfuric acid;
• sulfur dioxide – formed when concentrated sulfuric acid oxidizes the bromide ions;
• unreacted ethanol;
The purification sequence
Stage 1
If you have collected the underwater, transfer the contents of the collection flask to a separating funnel. Otherwise, pour the impure into the separating funnel, add some water and shake it.
Pour off and keep the layer.
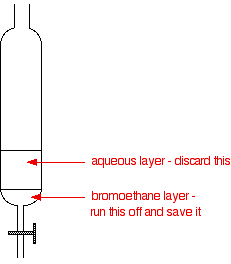
The water you discard will contain almost all of the hydrogen bromide, and quite a lot of any bromine, sulfur dioxide and ethanol present as impurities.
Stage 2
To get rid of any remaining acidic impurities (including the bromine and sulfur dioxide), return them to the separating funnel and shake it with either sodium carbonate or sodium hydrogencarbonate solution.
This reacts with any acids present liberating carbon dioxide and forming soluble salts.
Separate and retain the lower bromobutane layer as before.
Stage 3
Now wash the bromobutane with water in a separating funnel to remove any remaining inorganic impurities (excess sodium carbonate solution, etc). This time, transfer the lower bromobutane layer to a dry test tube.
Stage 4
Add some anhydrous calcium chloride to the tube, shake well and leave to stand. The anhydrous calcium chloride is a drying agent and removes any remaining water. It also absorbs unreacted butanol, and so any remaining ethanol may be removed as well (depending on how much calcium chloride you use).
Stage 5
Transfer the dry bromobutane to a distillation flask and fractionally distill it, collecting what distils over at between 35 and 40°C.
If there is any butanol l left that hadn’t been absorbed by the calcium chloride, that would certainly be removed because its boiling point is much higher.
Decant: gradually pour from one container into another, especially without disturbing the sediment.
Common Questions
How will the appearance of the iodoethane be changed by the addition of anhydrous calcium chloride in step 8?
Answer: cloudiness will disappear
How would the iodoethane be separated from the calcium chloride after step 8?
Answer: decant
It is not possible to effectively produce iodoethane by reacting ethanol with a mixture of sodium iodide and 50% sulfuric acid. This is because the sulfur in the sulfuric acid can be reduced to form substances such as hydrogen and sulfur. State what happens to the iodide ions in the sodium iodide when this occurs
Answer: iodide ions are oxidized to form iodine
The use of the beaker of cold water in step 1, and the slow addition of concentrated sulfuric acid in step 2 both prevent a reaction that gives unwanted inorganic products.
Identify one of those unwanted products, state the type o reaction occurring when those products form.
Answer: Product: bromine, Type of reaction: oxidation
How could the water layer be removed from the small beaker in step 5 without transferring the organic product?
Answer: Syringe to remove upper aqueous layer
Name the apparatus you would use to carry out the washing of the crude 1-bromobutane in step 6
Describe how would you obtain the organic layer from this mixture
Answer: using separating funnel
What change would you see in the appearance of the organic liquid when it is dried in step 4?
Answer: liquid would go clear
What would you see if concentrated sulfuric acid was added to solid sodium iodide? Give two observations
Answer: purple fumes, black grey solid formed
Explain why sodium bromide and sulfuric acid are required in step 2
Answer: to produce HBr
What is meant by decanting the liquid
Answer: To pour off the liquid leaving the solid behind
Suggest one advantage of using glass wool, rather than filter paper, when removing the calcium chloride in Step 5
Answer: Glass wool is less absorbent
Revise IAL Chemistry unit 3 within 45 minutes & get above 90%
- No BS, to the point, quick, and easy to revise.
- Actual common questions with answers from the question paper. Get easy marks in the bag during your exam.
- Questions are divided into different topics.
- A brief discussion on each topic to get your concepts clear.
- Available in kindle ebook and paperback formats on Amazon
Miscellaneous question-answers:
What additional step after heating strongly for five minutes is needed to make sure that all of the water of crystallization is removed?
Answer: Heat to constant mass
The enthalpy change obtained from this experiment is much less negative than the data booklet value suggest one likely reason for this difference other than a measurement error
Answer: heat loss, specific heat capacity is not 4.18
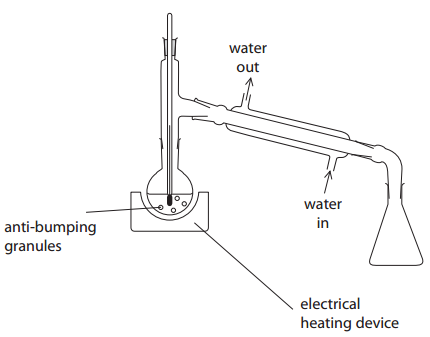
One of the errors is that the flow of water in the condenser is the wrong way round. Explain the effect of this error.
Answer: Condenser doesn’t fill properly/airlock forms
A further test is carried out on the mixture formed in b(i). This confirms that Y contains chloride ions, and not bromide or iodide ions. Describe this test and give your result.
Answer: Test: Add dilute ammonia solution, result: precipitate dissolves
What would you observe when the mixture formed in b(ii) is left to stand in sunlight
Answer: Goes dark, silver forms
Suggest why less gas is collected than expected, you should assume that the reaction is complete and no gas escapes.
Answer: Some CO2 dissolves in water
A student attempted to determine the molar mass of other carbonated in group 2 by the method used in this question. The student measured the volume of gas produced by each carbonate, but replaced the hydrochloric acid with sulfuric acid.
Explain why the results of the student’s experiments would give very inaccurate values for the molar mass of some carbonates of group 2.
Answer: Some group 2 sulfates are insoluble in water
Titration number 1 was a rangefinder, or rough titration. Describe how you would use the rough titration value when carrying out accurate titration
Answer: Add slowly when approaching rough value
Without the reflux condenser, the procedure in step 2 would become more hazardous. Explain why
Answer: to prevent escape of flammable liquid
A few drops of concentrated sulfuric acid were added to a small portion of solid x in a test tube, misty fumes, but no other vapors were seen.
Identify the fumes by name or formula
Answer: Hydrogen Chloride gas
Describe a further chemical test to confirm the identity of the gas responsible for the misty fumes. Give the expected result of the test.
Answer: Test: with ammonia gas, result: formation of white fumes
Suggest two ways, other than improvement in insulation or use of more accurate measuring instrument, which would ensure that the measured temperature change was the maximum possible for the amount of reactants used.
Answer: Stir mixture, Measure temperature before adding magnesium hydroxide and for some after
Suggest the main hazard when using concentrated phosphoric(v) acid in this preparation
Give the precaution which would be taken when using it, other than the use of safety goggles and laboratory coat
Answer:
Hazard: corrosive
Precaution: wear goggles
Describe two ways in which this apparatus must be modified for safe and efficient use in Step 2. Assume the apparatus is suitably clamped.
1) Move the bulb of the thermometer to the opposite opening of the condenser
2) leave a gap between the condenser and receiving flask
Using the same equipment together with a stop clock, suggest a procedure that would improve the accuracy of this experiment by obtaining a more accurate temperature change. You must use the same mass of zinc powder and the same volume of 1 mol/dm3 copper(II)sulfate solution
Answer: Measure the temperature of CuSO4 every minute, for several minutes, plot a graph of temperature vs time graph
Suggest why the first washing of the product in step 7 is with dilute sodium thiosulfate solution rather than with water alone
Answer: Removes residual iodine
Explain why the temperature falls when more ammonia solution is added after the end point?
Answer: no further reaction occurring
Explain how the process of heating under reflux works and why it is often necessary to heat under reflux as in step
Answer: used to heat volatile liquids, prevents escape of reactants, so complete reaction occurs
Draw a fully labelled diagram of the apparatus used for suction filtration
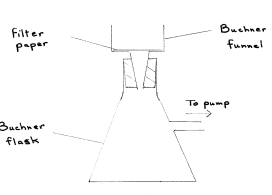
State two advantages of suction filtration over thermal filtration
Answer: faster, dries solid
Draw a fully labelled diagram of the apparatus you would use to determine the melting temperature of the 2 ethanoyl benzone acid crystals.
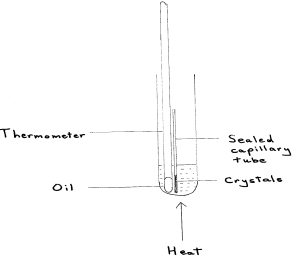
Describe two thin you could do to ensure that the burette readings are as accurate as possible. Assume that the burette has been appropriately rinsed and filled with sodium thiosulfate solution
Answer: measure level at the bottom of the meniscus, have eyes level with the meniscus
Explain why a stopper should not be placed at the top of the condenser as shown in diagram 1
Answer: to prevent explosion
Catalyst is not used up during a reaction, outline an experiment to demonstrate that manganese(IV)oxide is not used up in the decomposition of hydrogen peroxide
Answer: weigh before reaction, dry, filter after reaction, reweigh
Suggest why in both the distillations the receiver is immersed in ice-cold water
Answer: The products are volatile
Reminder: you can get the extended version of these common questions and answers Edexcel IAL Chemistry Unit 3 Common Questions and Answers: Study and Revision Guide from Amazon in kindle or paperback formats.
Conclusion
Thank you for reading this blog! Hope you found the IAL Chemistry Unit 3 question answers very useful. I will try my best to continuously update this blog to provide you with the best information possible.
If you have any questions or suggestions then please feel free to leave a comment below.
Also please check out my other blogs, you might find something useful!



Thank , I would like you to proceed to give me update
Could please create one for unit 4 and unit 5?
Okay, I will try. Thank you
For this unit 3 are there any practical Questions I’m just slightly confused
These are for the written part
oh I see thank you very much
It was really helpful, thank you so much
Most welcome!
These were super helpful would you please make for unit 4, 5, and 6??
Thank you! I will try to make for those units.
thank you, this will help a lot to the students.
thank you so much!! this was so helpful 🙂
Most welcome dear, how did your exam go?
hey can u make add these questions in a pdf and share them? that would be better