When you start learning about organic chemistry for the very first time, the very first thing you will learn the concepts of hydrocarbons. Like, what a hydrocarbon is, its classification, general formula, and so on. Hydrocarbons and their derivatives are the core of organic chemistry.
So firstly, let’s define a Hydrocarbon:
A Hydrocarbon is a compound that is made of hydrogen and carbon only.
Let’s get one thing clear, a hydrocarbon is a compound made out of only Hydrogen and Carbon atoms. So if there are any other atom(s) of any other elements present in the compound, then it is not a hydrocarbon. Please have a look at the Simple Example below:
Compound 1
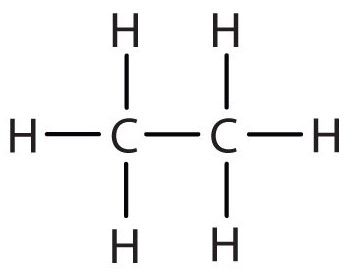
- Is a Hydrocarbon: This compound is made of Hydrogen and Carbon ONLY.
Compound 2
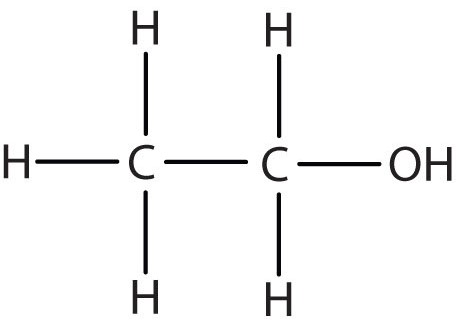
- Not a Hydrocarbon: Because apart from carbon and hydrogen it also contains an oxygen atom.
In this blog, I am going to discuss some of the important concepts of Hydrocarbons, their classification, their general formula, homologous series, how to name them. Also covering some important concepts about one of the most important derivatives of Hydrocarbon, which is Alcohol.
What will this blog cover and what it will not
Before you start reading this blog, let me make it clear what this blog will cover and what it will not. So please have a look at the table below, which shows the topics this blog will cover vs what it will not.
In the initial part of this blog, I have already discussed what a hydrocarbon is, I have also shown how to differentiate between a hydrocarbon and a non-hydrocarbon. If you’ve missed this part, then please CLICK HERE, to review that part again.
In case you are a complete beginner in organic chemistry, I would also recommend you to read this book “Organic Chemistry I For Dummies (For Dummies (Lifestyle))“
THIS BLOG WILL COVER
- Definition of Hydrocarbon
- Differentiating between Hydrocarbon and non-hydrocarbon
- Classification of Hydrocarbons
- General Formulas of Homologus series
- Homologous Series
- Naming Hydrocarbons
- Touching up on a derivative of hydrocarbon
THIS BLOG WILL NOT COVER
- Structural isomerism
- Covering indepth about derivatives of Hydrocarbons
- Reactions of derivatives of hydrocarbons
- Polymerization
- Extraction of crude oil, cracking
- Polymerization
A Closer Look at Carbon and Hydrogen atoms
Hydrocarbons are covalent compounds. The carbon and hydrogen atoms covalently bond with each other to form a hydrocarbon. If your concepts about covalent bonding are not clear, then you can have a look at my blog “What is Covalent Bonding and How it is formed“.
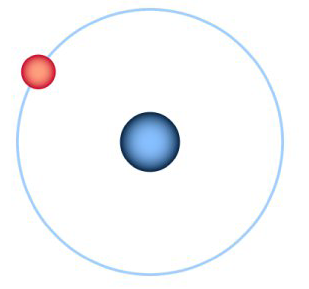
Carbon Atom: The carbon atom has four unpaired electrons in its outer shell. This means that it can form up to four covalent bonds. The diagram on the right with four lines shows that carbon is available to form four covalent bonds. Four atoms could attach themselves to a carbon atom by doing a covalent bond with them.
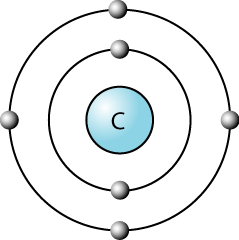
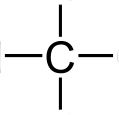
Hydrogen Atom: compared to a carbon atom, a hydrogen atom has only one electron in its outer shell. Hence it can form only one covalent bond. So a hydrogen atom can attach itself to carbon by doing a simple covalent compound. The single line beside the hydrogen atom represents that it can form only one covalent bond with the carbon compound.
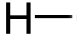
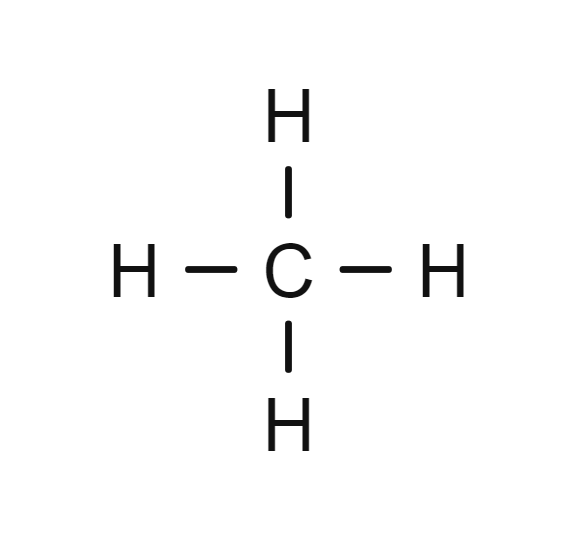
Example of the Simplest Hydrocarbon
The most simple hydrocarbon consists of only one carbon atom. As the carbon atom can form 4 covalent bonds, so it bonds covalently with 4 other hydrogen atoms. And this is the product below which is formed. Its name is methane. Do not worry about the naming too much, I will cover that part in great detail later on in this blog.
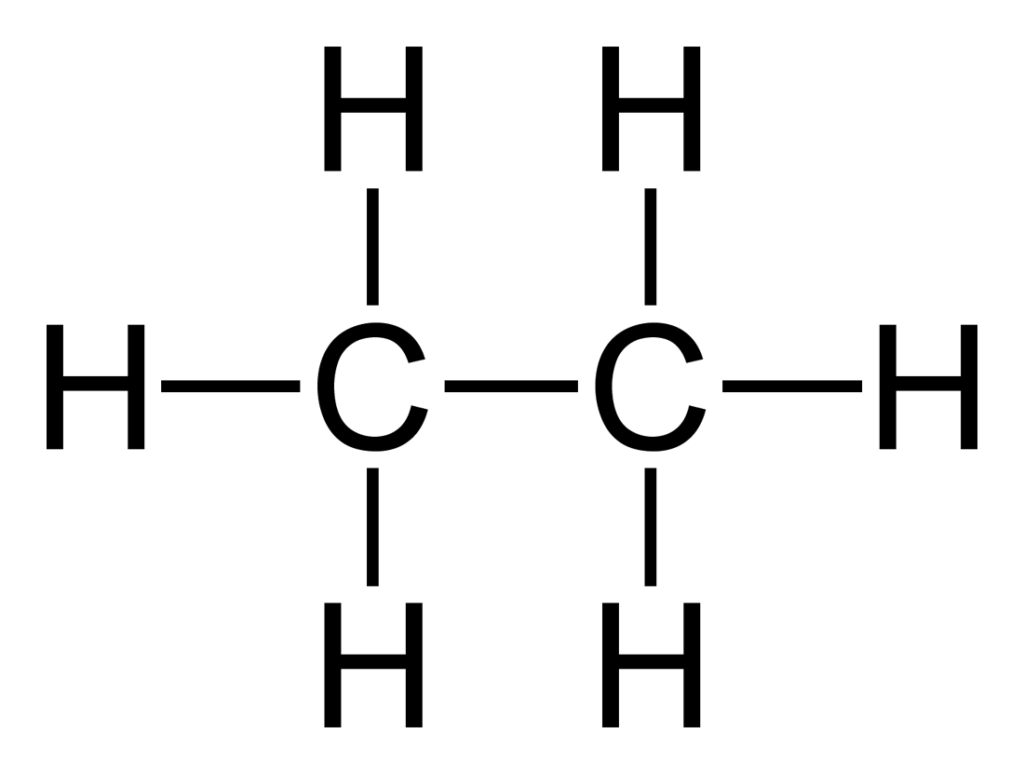
Now let’s have a look at an example showing a hydrocarbon with two carbon atoms. The name of this hydrocarbon is ethane.
Classification of Hydrocarbons
There are many types of hydrocarbons, but in IGCSE chemistry we will be dealing with only 2 of them, Alkane and Alkene. Plus I will cover some concepts about a derivative of hydrocarbons called Alcohols.
Alkane is the simplest type of hydrocarbon, in this type of hydrocarbon the carbon atoms simply join up with as many hydrogen atoms as possible. Each carbon atom forms four covalent bonds. Please have look at two Alkanes below, methane and ethane.


An Alkene is also a hydrocarbon, but it has one carbon to carbon double bond (C=C) in its structure. This means it doesn’t use up all its unpaired electrons to bond with hydrogen. There is no alkene with only one carbon atom because the alkene needs to have at least two carbon atoms for carbon to carbon bond to exist. Below are the examples of two alkenes, ethene(2 carbon atoms), propene(3 carbon atoms).
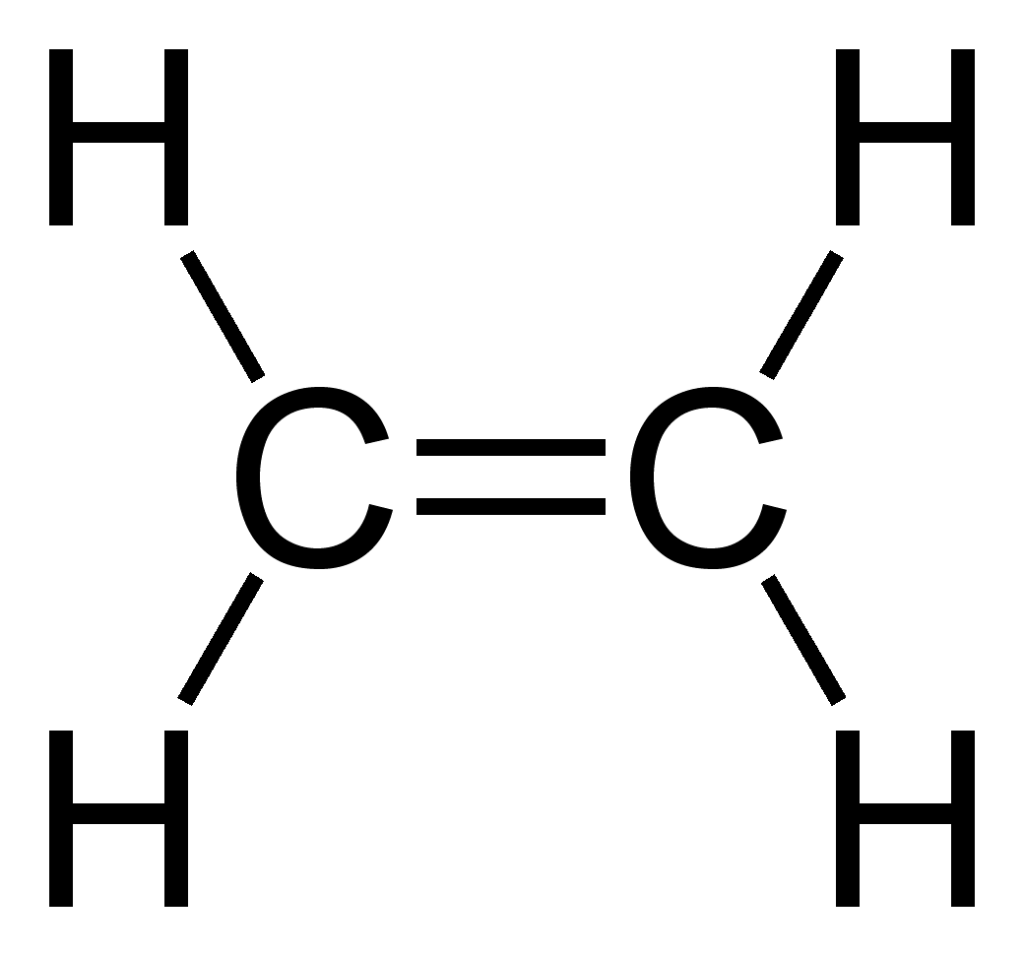
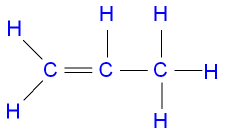
Derivatives of Hydrocarbons
There are many derivatives of hydrocarbons, hydrocarbon derivative means that the compound is formed from a hydrocarbon but is not a hydrocarbon anymore as it contains atoms from other elements other than Carbon or Hydrogen. Some hydrocarbon derivatives are natural and some are synthetic. But in this blog, I am going to touch up one of the most important derivatives of hydrocarbons, which is Alcohol.
The structure of Alcohol is very similar to that of alkanes, no carbon to carbon double bond is present in its structure. The only difference is that one hydrogen atom is replaced by an -OH group(will discuss Functional group later on this blog), please have a look at a simple 2 carbon alcohol structure below, it is called ethanol. Alcohol is not a hydrocarbon as it contains an oxygen atom apart from the usual carbon and hydrogen atoms. You can call simply call it a derivative of hydrocarbon. To learn how alcohol is obtained from sugarcane, you can have a look at my blog “Production of Ethanol From Sugarcane“.
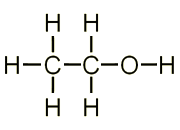
General Formulas
What is General Formula? With the help of the general formula, we can obtain the molecular formula of a specific hydrocarbon, given that we know the number of carbon atom(s) present in the hydrocarbon. Now you might ask that what is a molecular formula? The molecular formula simply tells us the number of atoms present from each element. For example, C2H6 is a molecular formula, it tells us that the compound has 2 carbon atoms and 6 hydrogen atoms in it. Of course, you can draw a hydrocarbon and count the number of carbon and hydrogen atoms present, but that would be a massive waste of time right? Imagine if you were told to write down the molecular formula of a hydrocarbon with 10 carbon atoms!
In the table below, are listed down the general formulas of Alkane, Alkene, and Alcohol
| Hydrocarbon / Derivative of Hydrocarbon | General Formula |
| Alkane | CnH2n+2 |
| Alkene | CnH2n |
| Alcohol | CnH2n+2O |
Examples:
Now, let me show you how you can use the General Formula to find out the molecular formula of each type of hydrocarbon.
Example 1: Finding the molecular formula of an Alkane with 3 carbon atoms
The General Formula of Alkane is CnH2n+2, the number of carbon atoms is 3 so n is 3, so the number of hydrogen atoms are 2(3)+2, which is 8. Hence the molecular formula for this alkane is C3H8.
Example 2: Finding the molecular formula of an Alkene with 4 carbon atoms
The General Formula of Alkene is CnH2n, the number of carbon atoms are 4 so n is 4, hence according to the formula, the number of hydrogen atoms are 2(4). Therefore the molecular formula for this alkene is C4H8.
Example 3: Finding the molecular formula of an Alcohol with 6 carbon atoms
The General Formula of Alcohol is CnH2n+2O, the number of carbon atoms is 6 so n is 6, hence according to the General Formula, the number of hydrogen atoms are 2(6)+2= 14. Hence the molecular formula of alcohol with 6 carbon atoms is C6H14O
Functional Group
The Functional Group in a hydrocarbon or derivative of hydrocarbon is an atom or group of atoms that gives the compound unique chemical and physical properties. For example, the functional group in Alkene is the carbon to carbon double bond, the function group in Alcohol is the -OH group, while Alkane does not have any functional group.
Homologous series
The Homologous series is a family or group of hydrocarbons or derivatives having the same functional group, same general formula, and similar chemical properties. They also have a similar trend in physical properties. For example, Alkane is a homologous series, Alkene is a homologous series, Alcohol is also a homologous series. There are also a bunch of other homologous series such as carboxylic acid, aldehydes, and so on, but you do not need to focus on these ones. In this blog, we are only going to focus on Alkane, Alkene, and Alcohol.
Naming a Hydrocarbon
Naming a hydrocarbon and understanding what the name means is one of the most important concepts of organic chemistry. The name of a hydrocarbon mainly depends on the number of carbon atoms and in which homologous series the hydrocarbon is in. There are also other factors in the naming such as structure, isomerism, etc. but for now, I am going to ignore these factors to simplify things, as this blog is mainly for beginners who are starting out with organic chemistry. But if you want more in-depth information about naming organic compounds along with different structures, then please have a look at my blog “Best IGCSE Organic Chemistry Notes“.
The name of a hydrocarbon is made out of two parts, the PREFIX, and SUFFIX. The prefix part comes first and is then followed by the suffix.
Name of Hydrocarbon = PREFIX + SUFFIX
The prefix part represents the number of carbon atoms the hydrocarbon has, while the suffix part represents that to which homologous series the hydrocarbon belongs. Before we go on and start naming some hydrocarbons, let’s have a look at the table of prefixes and suffixes. Only in the case of alcohol, there is an “an” in-between the prefix and suffix, it will be clarified to you in the examples below.
PREFIX lists representing the number of carbon atoms
| No. of Carbon Atoms | PREFIX |
| 1 | Meth- |
| 2 | Eth- |
| 3 | Prop- |
| 4 | But- |
| 5 | Pent- |
| 6 | Hex- |
| 7 | Hep- |
| 8 | Oct- |
| 9 | Non- |
| 10 | Dec- |
SUFFIX representing Homologous series
Here I am providing only the suffix of alkane, alkene, and alcohol as we are dealing with only three homologous series in this blog.
| Homologous series | SUFFIX Name |
| Alkane | -ane |
| Alkene | -ene |
| Alcohol | -ol |
Examples
Now let’s have a look at some examples so it will be clear to you how to use the prefix and suffix to name a hydrocarbon.
Example 1: Naming an Alkane with 6 carbon atoms. Please refer to the prefix and suffix table above.
Here the number of carbon atoms is 6, so the Prefix is “Hex”
The homologous series is Alkane, so the suffix is “ane”
Name of hydrocarbon = Prefix + Suffix = Hex + ane = Hexane
Example 2: Naming an Alkene with 5 carbon atoms. Please refer to the prefix and suffix table above.
Here the number of carbon atoms is 5, so the Prefix is “Pent”
The homologous series is Alkene, so the suffix is “ene”
Name of hydrocarbon = Prefix + Suffix = Pent + ene = Pentene
Example 3: Naming an Alcohol with 8 carbon atoms. Please refer to the prefix and suffix table above.
There is an “an” in-between the prefix and suffix when naming an alcohol
Here the number of carbon atoms are 8, so the Prefix is “Oct”
The homologous series is Alcohol, so the suffix is “ol”
Name of hydrocarbon = Prefix +”an”+ Suffix = Oct +”an”+ol = Octanol
Conclusion
So in this blog, we covered the basic to advanced concepts about hydrocarbons. Now we know the different classifications of hydrocarbons, Alkane, and Alkene. We also learned a few things about a derivative of hydrocarbon called Alcohol. We learned in great detail about general formulas, homologous series, and naming different kinds of hydrocarbons.
If this blog helped you out, then please feel to share this blog with your friends who might need help with Organic Chemistry. Also please have look at other blogs on this website, you might find something useful.

I was thinking that organic chemistry is a really, really hard chapter, therefore I almost just gave up on it, but honestly, your blog literally saved me, it explains everything in details yet as simple as possible so that a student would feel somewhat reassured they will learn while reading it, I have noted down everything you explained word to word to refer to them in future revision, thank you so much, truly you will make me pass if god wills!
Thank you Mena for the wonderful feedback. Comments like these encourage me to write more blogs like this. Please also check out my other blogs, you will also find them useful. Please share this blog with your friends who need help with organic chemistry via Facebook, Twitter, or directly emailing them. Good luck with your exam.
I found your blog really helpful but I had a question in some books I referred to the general formula for alcohols also included H (CnH2n+2OH). Could you tell me what formula am I supposed to refer to?
Thanks