Importance of Metals
Metals are everywhere. Look around you and try to find something which contains no metal at all! Even the latest iPhone and Samsung phones have an
It is very hard to imagine life without metals. Before we start to discuss how to obtain metals from their ores, we are going to learn about their uses first. You would be more interested to know how industries extract metals once you are
Uses of Metals
Let’s learn about some metals and their uses. There are many metals in this universe but we only use a few of them! We are simply going to discuss some of the common metals and their uses. The important ones are copper, iron, aluminum. So let’s get started!
Copper: Copper is an amazing metal. All the electricity and you are enjoying now is due to copper. The electricity cables are made of copper. Copper is even embedded inside electrical devices such as power circuit boards to conduct electricity. In some countries, copper is used to making hot water pipes as they do not react with water. The coaxial cable of your TV also has copper inside it.

Iron: Pure iron does not have a variety of uses. But alloys of iron such as steel are highly useful. Alloys are nothing but a mixture of different types of metals. Alloys of Irons are used to make cars, nails, bridges etc. We are going to discuss in details about the different types of iron in the extraction of the iron part.
The Aluminium metal is extracted from it’s ore Bauxite by a method called Electrolysis. We are going to have an in-depth look later on this blog of how Aluminium metal is ectracted.

Metal such as gold and silver are usually used to make pieces of jewelry. Though gold and silver are better electrical conductors than copper but copper is used instead as it is cheaper.
The Reactivity Series
Before we even start scratching the surface of the top of extraction of metals from their ores. We must clear up our concept about the reactivity series. Because of all the concepts of metal extraction is based on this. Firstly let’s have a look at the reactivity series. Keep note about the position of the following elements iron, aluminum, and carbon.
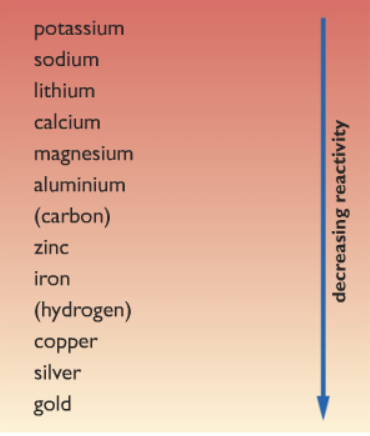
Note that iron is less reactive than carbon while aluminum is more reactive than carbon. From where come carbon come from? You will know in a while 😉
Get this concept clear about the displacement reaction, a more reactive metal is able to displace a less reactive metal. But a less reactive metal is not able to displace a more reactive metal.
Suppose, let’s have look at an example to have a better understanding. A is a more reactive metal, B is a less reactive metal and C is the anion.
A + BC → AC + B
A displaces the less reactive metal B
But this thing won’t be possible the other way round as B is less reactive than A.
B + AC → No Reaction
Now let’s have a look at a real displacement reaction.
Zn(s) + FeCl2(aq) → ZnCl2(aq) + Fe(s)
Here, solid zinc metal displaces iron from aqeous solution of iron(II) chloride because zinc is more reactive than iron.
If it’s the other way round, then the reaction won’t take place. Because less reaction iron is not able to displace more reactive zinc.
Fe(s) + ZnCl2 → No reaction
I hope this concept is clear because both the extraction of Iron and Aluminum uses the concept of the reactivity series.
If you are still finding it difficult to grasp the concept of Reactivity series, then check out this cool Reactivity of metals Poster on Amazon. This poster contains colorful charts and diagrams which help you understand the concept of Reactivity series better.
Extraction of Metals
Industries extract metals from their ores. What are ores? Let me explain in a simple way. Usually, less reactive metals such as gold and silver are found in their pure form in the soil. But this is not the case with more reactive iron or aluminum. Usually iron and aluminum exist as compounds, with oxygen, also known as oxides. Some other mineral or other compound is also present. But the majority is a metal oxide. In conclusion, in most cases, ores are simply metal oxides along with other small amounts of minerals.
But not in all cases ores are metal oxides. For example, ZnS, Zinc Sulfide or also known as zincblendes is an ore of zinc.
Extraction of Iron
Iron is a fairly reactive metal, hence we cannot find iron in its pure form. We have to obtain iron from its ores. Iron has three ores from which we can obtain it from.
The three ores of iron are:
- Pyrite (FeS2)
- Magnetite (Fe3O4)
- Haematite (Fe2O3)

Industries usually use Haematite to extract iron from, but they can also use Magnetite too. Here we are going to see an example of the extraction of iron from Haematite.
To have iron from haematite we must remove the attached oxygen. We can do this by a displacement reaction. We can react a more reactive metal such as zinc with haematite, which will displace iron from the oxygen. Then it will be possible to collect the iron.
But if we use zinc to extract iron, then the zinc is gone! Remember we need to extract zinc too from its ore to extract iron. This does not make sense, it is like making cakes with cupcakes, we need to make the cupcakes first in order to make the cake ( ͡° ͜ʖ ͡°)
But carbon, on the other hand, is a better alternative. Carbon is more reactive than iron, plus also it is cheaper and available in plenty. Carbon comes in the form of coal, in industrial level, it is also known as coke. Please do not mistake this coke as the soft drink coca cola, or cocaine drug. The carbon in the coke reduces the iron(III)oxide to iron. But the carbon does not reduce it directly, but in several steps, I will discuss that later on this blog.
In order to extract iron industries use a structure called Blast Furnace. Have a look at the diagram below:
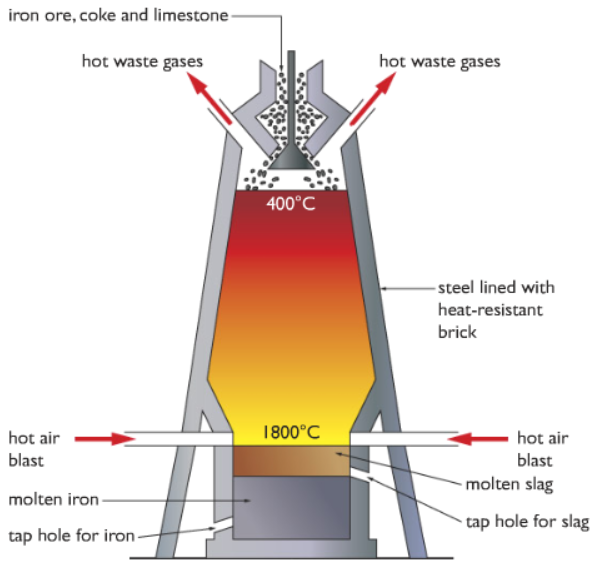
Looks scary and difficult right?! It might look scary but not a difficult concept to digest. I will be explaining this process step by step so it will be clear to you.
Step 1: Firstly the raw materials iron ore (Haematite, Fe2O3), coke (carbon) and limestone (calcium carbonate, CaCO3) is added from the top of the blast furnace. I will discuss the reason for adding limestone later on. Just to give a simple idea, for now, it helps to remove some of the impurities.
Step 2: As you can see from the diagram, hot air is blasted from both sides at the bottom. This heats up the inside of the blast furnace.
This heat causes the oxygen in the hot air to react with the carbon in coke.
C(s) + O2(g) → CO2(g)
The reaction between carbon and oxygen is highly exothermic. This releases more heat inside the blast furnace. So the reaction between carbon and oxygen is mainly used to generate heat.
Note: The body of the blast furnace is covered with heat-resistant bricks. This ensures that a minimum amount of heat is lost through the conduction process.
Step 3: The extra heat energy released from the above reaction increases the temperature inside the blast furnace up to 1800 °C. This high temperature causes carbon dioxide to react with oxygen in the air to produce carbon monoxide(CO).
CO2(g) + C(s) → 2CO(g)
Step 4: Carbon monoxide is a powerful reducing agent. This carbon monoxide reduces the iron(III)oxide to iron metal.
Fe2O3(s) + 3CO(g) → 2Fe(l) + 3CO2(g)
But in some places of the blast furnace, the temperatures are very high. In those high-temperature regions, carbon directly reduces the ore to iron. The iron settles at the bottom of the furnace in a molten state. It is impure too.
Fe2O3(s) + 3C(s) → 2Fe(l) + 3CO(g)
Function of Limestone
As I mentioned before, the limestone(CaCO3) helps to remove impurities. The main impurity in this process is sand or Silicon dioxide SiO2
Under the heat of the blast furnace, the limestone decomposes to form carbon dioxide and calcium oxide.
CaCO3(g) → CaO(s) + CO2(g)
The calcium oxide then reacts with silicon dioxide to form calcium silicate.
CaO(s) + SiO2 → CaSiO3
The calcium silicate is also known as slag. It is in molten form and floats above the molten iron.
Please refer to the blast furnace diagram above.
The bottom tap is for iron, from that tap iron is extracted.
The tap above is for slag, from that tap slag is extracted.
Different types of iron, their properties and uses
There are five different types of iron Cast iron, Mild Steel, Wrought Iron, High-carbon steel, Stainless steel. It is based on their amount of carbon content they have. Carbon is used to extract iron, so some carbon remains with the iron metal.
Cast Iron: Cast iron is the metal which is directly obtained from the blast furnace without any processing. It is also known as pig iron. Cast iron is very impure, it contains 4% carbon. Cast iron is extremely brittle, hence it has limited uses.
Some uses of cast iron:
- Manhole covers
- Guttering and drain pipes
- Cylinder blocks in car engines
Mild Steel: Mild steel contains 0.25% carbon. Hence it is more malleable and quite hard at the same time. Hence it has a much wider range of uses than cast iron.
Some uses of Mild Steel:
- Car bodies
- Ship Building
- Nails
- Wires
- Girders
- Bridges
Wrought Iron: Wrought iron is pure iron. This makes the iron highly malleable. Though it is easier to bend it into different shapes, too much malleability means it has less strength. Hence it is not used for structural purposes.
Wrought iron is mainly used for decorative items such as gates, railings etc.
High Carbon Steel: High carbon steel has a carbon content of 1.5%. This makes it hard and brittle.
Uses of High Carbon Steel:
- Cutting tools
- Masonry Nails (the type of nails which are hammered into concretes)
Stainless Steel: Stainless steel is an alloy of iron, chromium, and nickel.
Some uses of stainless steel are:
- Kitchen sinks
- Saucepans
- Knives, forks, spoons
- Medical industry
- Chemical industry
Extraction of Aluminium
Aluminium is a fairly reactive metal, hence we cannot find this in its pure form. It is in fact found in the earth crust from its ore, called Bauxite. Bauxite is simply impure aluminium Oxide (Al2O3).
Let’s review the reactivity series again:

From the reactivity series, we can see that aluminium is more reactive than carbon. Hence we cannot use the same method as with iron to remove the oxygen from aluminium. Because carbon is less reactive than aluminium, hence carbon is unable to displace aluminium.
Hence we have to use a process called Electrolysis. In this process, electrical energy is used to remove the oxygen from
Firstly, the molten the bauxite is dissolved in molten Cryolite at 1000 degree
After the bauxite is dissolved, the Al3+ ions and O2- ions are free to move around within the solution.
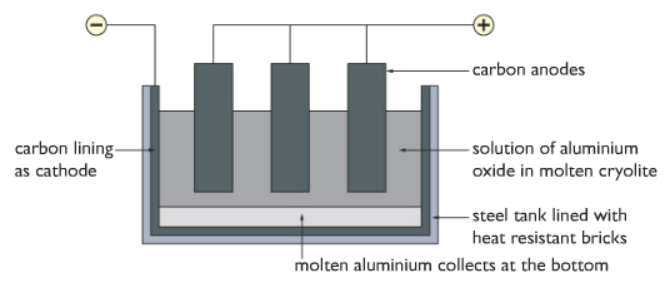
Both of the anodes and cathodes are made of graphite (carbon). The cathode covers the inner surface of the container.
Reaction at the cathode:
Al3+(l) + 3e– → Al (l)
The aluminium collects at the bottom of the container is liquid form.
Reaction at the anode:
2O2-(l) → O2(g) + 4e–
The oxygen gas produced at the anode escapes in gaseous form. But due to very high temperatures, the oxygen gas reacts with the anode (made of carbon) to form carbon dioxide gas. Hence the anodes need to be replaced continuously as it keeps getting used up.
To get even more advanced knowledge about the extraction of Aluminium, electrolysis process, and more details about the types of Anodes and Cathodes used, you can read this book “Refractories for Aluminum: Electrolysis and the Cast House” from Amazon.
Some Uses of Aluminium:
- Overhead wires
- Air crafts
- Cooking Utensils
Thank you so much for reading this blog about extraction of metals such as Iron and Aluminum.


Perfect.
Thank you very much!
i need a copy of the lecture sheet.