Bonding in Ionic Compounds
Ionic bond or even known as the electrovalent bond is formed by the gain or loss of electrons, the ionic bond structure is a regular arrangement of ions, the formation of ionic compounds can be shown using dot-cross diagrams. There is a high electrostatic attraction between the cations and anions for which they have a very high melting and boiling point. We are going to cover in more detail below.
Chemical bonds
The forces holding atoms together
Chemical bonds affect physical properties such as melting and boiling points
Whats an ionic bond
- Forms between metal and non-metal
- Oppositely charged ions are formed
- Strong electrostatic attraction between ions, the attractive force is known as ionic bonding
- The ions are arranged in an arrangement called ionic lattice
- In the ionic lattice there are both similarly and oppositely charged ions
- The arrangement in the ionic lattice maximize the attraction between the oppositely charged ions and minimizes the repulsion between the similar charged ions
- The electrostatic attraction between the ions are known as ionic bond
To learn more about ionic bonding in great detail, you can check out the page number 86 of the book “Chemical Structure and Bonding” from Amazon.
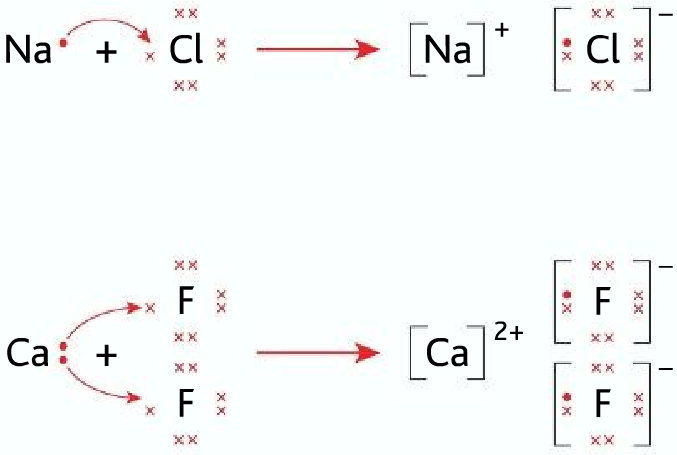
When sodium and chlorine reacts Na+ and Cl– ions are formed, but why not Na–, Cl+ or Na2+ ,Cl2-
Electronic Configuration
Na 1s22s22p63s1
Cl 1s22s22p63s23p5
Sodium atom can achieve a full outermost shell by losing the electron 3s1. A full outermost shell is highly stable. Its electronic configuration becomes that of Neon.
Na+ 1s22s22p6
Ne 1s22s22p6
Na+ and Ne are isoelectronic ( they have the same number of electrons and arrangements of electrons)
Chlorine also achieves a full outer shell by receiving one electron. Its electronic configuration becomes that of Argon.
Cl– 1s22s22p63s23p6
Ar 1s22s22p63s23p6
Cl– and Ar are isoelectronic
- The ionic bond is also known as electrovalent bond.
Dot-cross diagrams:
- To draw dot-cross diagrams follow the simple rules that you used to follow in O level
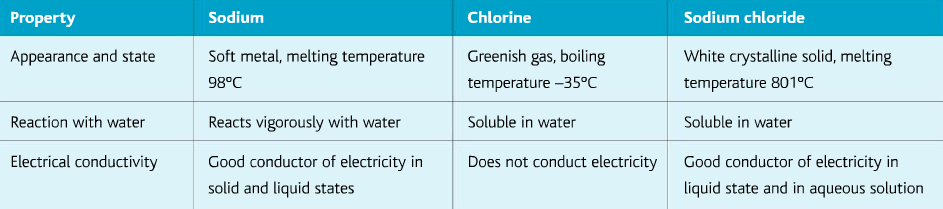
Giant Ionic Lattices:
When metals and non-metals react to form an ionic bond, the ionic compound is very different from the elements which reacted. As shown in the table:
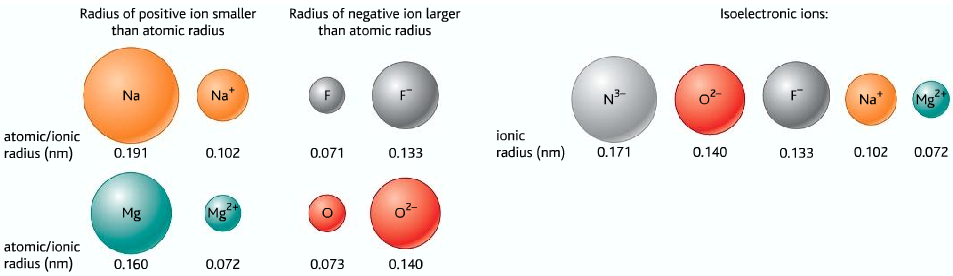
Trends in ionic radii:
- Positive ions are smaller than the original atom
- Negative ions are bigger than the original atom
- A sodium Chloride lattice structure is called Face Centered Cubic Structure, each ion is surrounded by 6 other ions of opposite charges, the coordination number of 6. One Na+ ion would be surrounded by 6 Cl– In turn one Cl– would be surrounded by 6 Na+ ions.
- Cesium ion is larger than the sodium ion, therefore it has a different lattice structure called Body Centered Cubic Structure. Each ion is surrounded by 8 other ions of opposite charges, coordination number 8.
Evidence for the existence of ions:
Physical properties
- Ionic substances have high melting and boiling points, this can be explained due to the existence of ions with high attraction between oppositely charged ions, which takes a lot of energy to overcome.
- An ionic compound can conduct electricity in molten or aqueous state but not in solid state. This can be explained in that it contains ions stuck in the lattice in a solid state but they are free in the liquid or aqueous state to move around and conduct electricity.
- Ionic compounds are brittle, this can also be explained that it contains ions. When force is applied, ions with like charges face each other, they repel and move apart.
Electrolysis
Existence of ions can also be showed by the electrolysis of copper(II)chromate(IV)
CuCrO4 (aq) à Olive green
Cu2+(aq) à blue
CrO42- à yellow
The positive Cu2+ is attracted to the cathode so a blue color is seen around the cathode.
The negative CrO42- is attracted to the anode so a yellow color is seen around the anode.
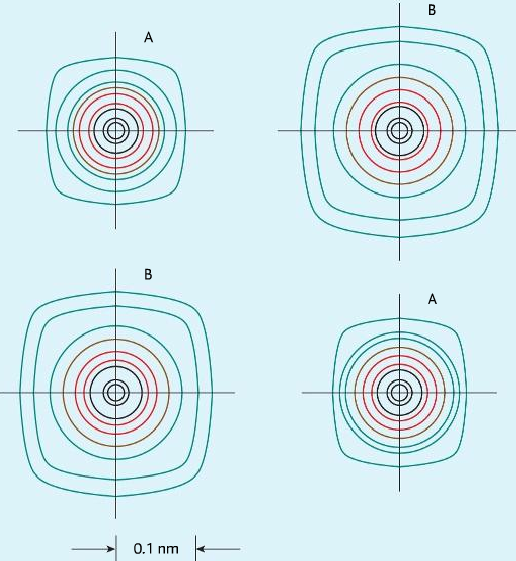
Electron density map
- It is a map created by passing x-ray through the ionic compound. Electrons diffract x-ray and produces bright spots.
- Different distances between ions show it is a lattice.