Gasoline and Diesel are two of the most important fuels in our daily life. Gasoline or in short called “Gas” is used to run cars, while diesel is used to run trucks, lorries, and other heavy machines. Both gas and diesel are obtained from crude oil by the “Fractional Distillation” process. If you are not clear of how they are obtained from crude oil, then please read the blog “How Gas & Diesel is Extracted from Crude Oil“.
Even though gas and diesel are obtained from crude oil by fractional distillation, still many major oil and gas companies use a method called cracking to artificially break down heavier hydrocarbons to produce even more gas/gasoline.
In this blog, I am going to discuss why most major oil and gas companies use cracking even though they obtain an amount of gasoline from crude oil. Also, I am going to explain that what is cracking and how it works.
NOTE: If your concepts in organic chemistry/ hydrocarbon are not clear, then please have a look at my in-depth blog “Organic Chemistry“. Simply keep that open in a new tab of your browser, so you can refer to it whenever you need to.
Gasoline is in much higher demand
When fractional distillation is done to crude oil, apart from gasoline there are other different fractions produced. Fractions like diesel, kerosene, Fuel oil, etc. Please have a look at the diagram below to have a look at the different fractions and their uses.
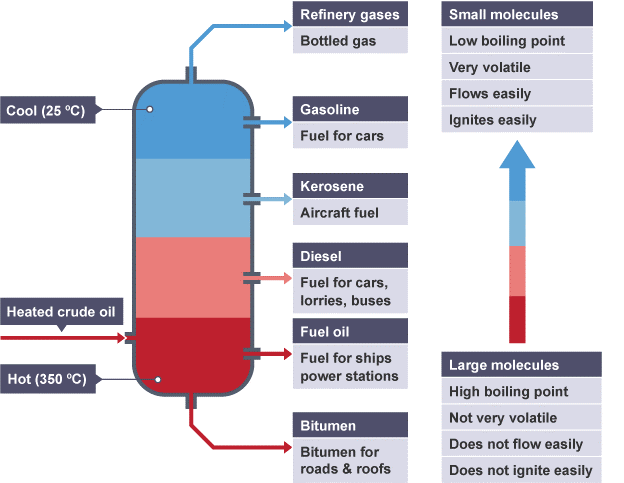
Gasoline is the most in-demand fuel due to its usage in cars. The amount of gasoline that is produced by the fractional distillation of crude oil is not enough to full fill the daily needs of the people.
Car engines cannot burn fuels such as Kerosene, Diesel, or Fuel oil because they consist of heavier hydrocarbons and is very difficult to ignite.
Therefore, to meet the high demand most major oil and gas companies use a chemical property called cracking to break down the heavy hydrocarbon fuels into Gasoline or lighter hydrocarbons, which can be burnt by small car engines.
What is Cracking & how it works?
Cracking is a chemical process, in which the large chain hydrocarbons are broken down to smaller hydrocarbons. At first, the heavy hydrocarbons are heated to convert them to gas. Then the mixture is passed over a catalyst of mixed silicon dioxide and Aluminum oxide at 600-700 ◦C. The process of cracking is very random, so when a hydrocarbon breaks, the products are not always the same. Have a look at the diagram below.
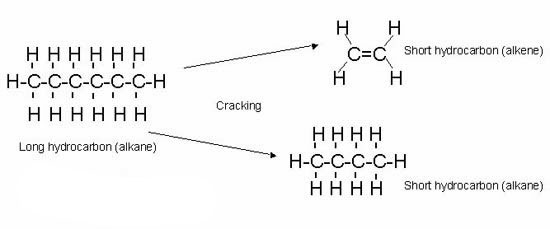
Cracking a hydrocarbon (alkane) produces one alkane and the rest are alkenes. Remember, that the number of carbon and hydrogen atoms will remain the same!
For example, a large alkane C13H28 can breakdown into several combinations of alkanes and alkenes
C13H28 → C2H4 + C3H6 + C8H18
C13H28 → 2C2H4 + C9H20
C13H28 → 2C2H4 + C3H6 + C6H14
C13H28 → 2C2H4 + C3H6 + C6H12 + H2
Conditions Required For Cracking
- Temperature : 600-700 °C
- Catalyst Used: Mixture of silicon dioxide and aluminum dioxide
- Pressure: 1 atm (normal atmosphearic pressue)
Conclusion
This is the reason why most major oil and gas companies use cracking to break down heavier hydrocarbons to gasoline. Please have a look at my other blogs, you might something very useful. Feel free to bookmark this post or share this with your friends who might need help with this topic.

