The Atomic Structure
The atom is the smallest particle, it is made out of smaller sub-atomic particles such as protons, neutrons, and electrons. The protons and neutrons are contained within the nucleus of the atom. The electrons are arranged in different shells or energy levels around the atom. In an atom there is an equal number of protons and electrons, therefore the atom is neutral.
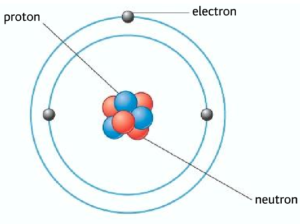
Proton and neutrons can be generally referred as nucleons
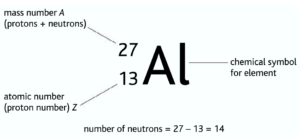
Isotopes: Atoms with the same number of protons but different mass numbers/neutron number. Isotopes have same chemicals properties because they have the same number of electrons.
But as their mass varies, their physical properties may vary. As a result, some isotopes may be unstable
Eg: carbon-12 ➝ stable and carbon-14 unstable
Ion: When an atom loses or gains electrons it forms an ion.
- Losing an electron forms a positive ion (cation)
- Gaining an electron forms a negative ion (anion)
Ionic bonding: Forms between gain or loss of electrons
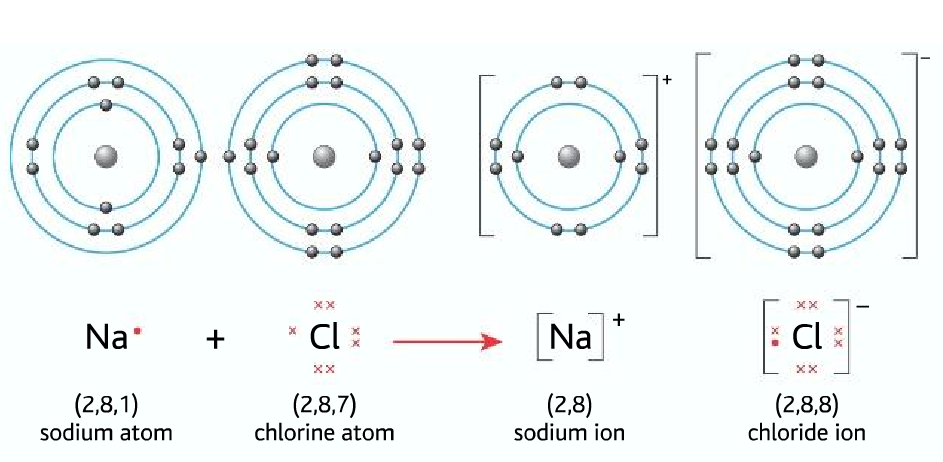
Ionic bonding forms between a metal and a non-metal
Covalent bonding (for more detail click here)
formed by sharing a pair of electrons
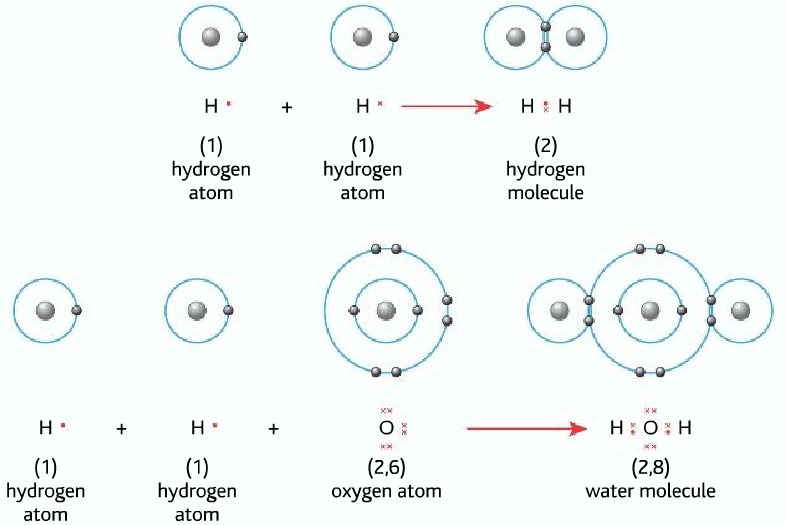
It is formed between non-metals
Diatomic
Two atoms of the same element existing as a molecule:
Eg: O2 , N2, Cl2, F2 , Br2, I2, H2
Monoatomic
Exists as a single atom. Especially noble gases because they can’t bond as their outer shell is already full-filled. Eg: Ne, Ar
Writing equations:
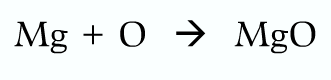
Wrong because oxygen should be diatomic
Correct:
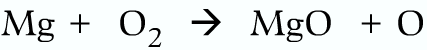
The total mass of the reactants and products should be same
Example 2:
Wrong:
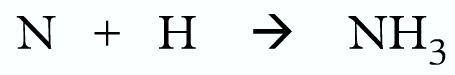
Correct:
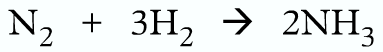
Element
A substance in which all atoms have the same number of protons
Molecule
Two or more atoms covalently bonded together
State Symbols:
s ➝ Solid
l ➝ liquid
g➝gas
aq ➝ aqueous (dissolved in water, not in any other substances)
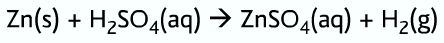
Ions in solution:
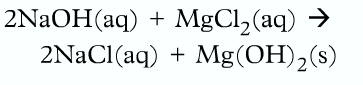
This type of equation is called the molecular equation because it shows the complete formula for every reactant and products.
Writing ionic equation:
Firstly breakdown into ions:
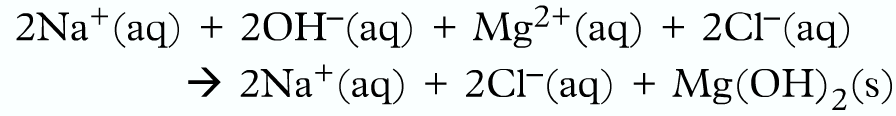
Then omit the spectator ions:
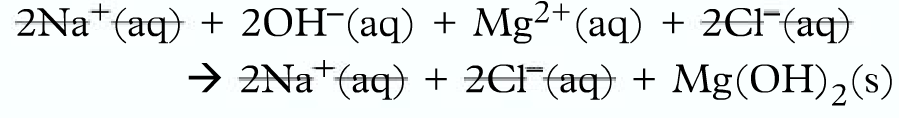
Finally, write the ionic equation:
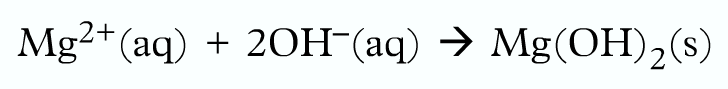
Working with numbers:
Very small or big numbers are expressed in the standard form:
1 000 000 ➝ 1 x 106 0.00015 = 1.5 x 10-4
17800 000 ➝1.78 x 107 3.1 10-3 = 0.0032
Relative Atomic Mass (R.A.M)
The relative atomic mass of an element is defined as the average mass of its isotopes compared with the mass of an atom of the carbon-12 isotope.
Suppose carbon-12
Hydrogen is 1/12 of the carbon-12, so hydrogen is 1 unit
Symbol of relative atomic mass (R.A.M) is Ar
Ar(Ca) = 40.1, meaning R.A.M of Ca is 40.1