In chemistry, we refer to Chemical bonding as a means or a way by which an atom attaches itself with other atoms. Basically, there are three types of chemical bonding in chemistry, and they are covalent bonding, ionic bonding, and metallic bonding. In this IGCSE chemistry chemical bonding blog post, I am going to cover the basic concepts of these three types of bonding. Please stick till the end. This blog post might be the reason you may get an A or A* in your IGCSE Chemistry Exam!
What is Covalent Bonding?
To say this in the simplest way possible, Covalent bonding is the bond formed by sharing a pair of electrons. So atoms stick together simply by sharing a pair of electrons.
How does Covalent Bonding Works?
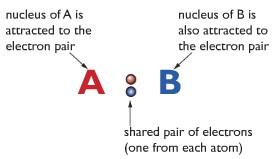
When atoms share a pair of electrons. The shared electron pair is in the middle of the two atoms. The shared electron pair has a high concentration of negative charge. The atoms (in this example A and B) have nuclei with a high concentration of positive charge. The shared electron pair is attracted to the positive nuclei of both the atoms. This is how it holds the two atoms together. The shared electron pair is also known as bond-pair in some cases.
Important reminder: In the shared electron pair, each electron comes from different atoms. But in cases where the shared electron pair comes from the same atom, that bonding is known as dative covalent bonding or also known as coordinate bonding. But for now, at this IGCSE Chemistry level, you can ignore dative covalent bonding.
To get more comprehensive knowledge about chemical bonding you can check out this amazing book “Chemical Bonding (Oxford Chemistry Primers)” on Amazon.
When an atom has unused electron pairs which are not used in covalent bonding. They are known as lone pairs.
Molecule: The molecule is a very important term in chemistry. We now know that an atom can attach itself with other atoms using covalent bonding. In cases where two or more atoms join together using covalent bonding to form a group. Then the group is known as a molecule. Some examples of molecules: CO2, SO2, H2o, etc.
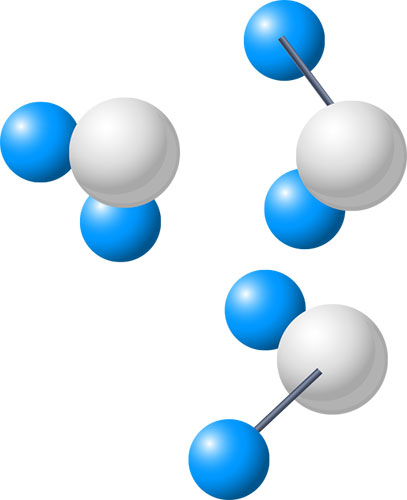
Diatomic: The term diatomic refers to a specific type of molecules. When two atoms from the same elements form a molecule, that is known to be a diatomic molecule. For example, two hydrogen atoms forming a molecule (H2 ) is an example of a diatomic molecule. Some other examples of diatomic molecules are: Cl2, Br2, O2
Explaining Dot-Cross Diagrams
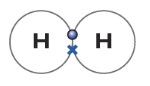
The dot-cross diagram is an essential part of explaining how covalent bonding works. A dot-cross diagram consists of nothing but circles, dots, and crosses. The dot represents electron(s) from one atom, while the cross represents electron(s) from the other atom. When one pair of the electron is shared, it means one covalent bond is formed. One covalent bond is represented by dot-cross.
Below is a simple example of dot-cross diagram between two hydrogen atoms. The cross represents an electron from one hydrogen atom while the dot represents an electron from another hydrogen atom. This molecule is diatomic as I explained before.
Sometimes it is necessary to quickly draw a molecule while showing the covalent bonds. But drawing the dot-cross diagrams every time might be a big waste of time. Hence there is an alternative way. We can represent a single covalent bond by a “—“ instead of dot-cross. The lone pairs can simply be represented using two dots.
Below is a simple example of an ammonia molecule and of how can we represent a dot-cross ammonia molecule using dashes.
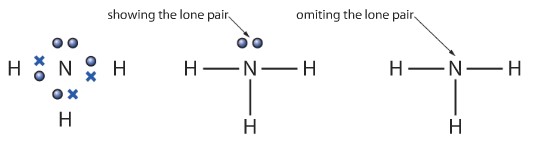
Some Examples of covalent compounds
Hydrogen molecule H2

The hydrogen atom has one electron. So it can form a covalent bonding with another hydrogen atom. As you can see in the figure above. The dot represents an electron from one atom, while the cross represents an electron from another atom. The Hydrogen molecule can be represented as H—H.
Hydrogen Chloride molecule (HCl)
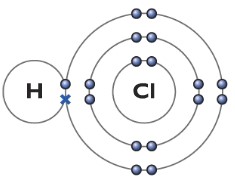
The hydrogen atom has one electron, while the chlorine atom has seven electrons in its outer shell. So chlorine needs only one more electron to full fill it’s outer shell. Among the seven electron, one of the outer shell electron in chlorine is unpaired. Hence chlorine uses that electron to form a covalent bond with the hydrogen atom. The cross represents the electron from the hydrogen atom, while the dot represents the electron from the chlorine atom. HCl can be represented as H—Cl.
Chlorine molecule Cl2
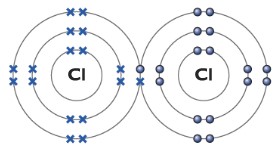
The molecule of chlorine consists of two chlorine atoms, it is a diatomic molecule. Each chlorine atom has seven electrons in its outer shell. Each one of the chlorine atoms needs one more electron to full-fill its outer shell. They simply do it by forming a covalent bond, sharing a pair of electrons. The dot represents electrons from one chlorine atom while the cross represents electrons from another chlorine atom.
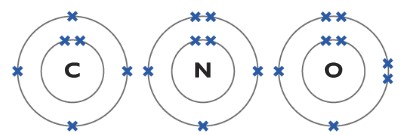
Understanding the structure of Carbon, Nitrogen and Oxygen atom
Now, lets learn some important concepts about carbon, nitrogen and oxygen atoms so it will be easier for you to figure things out!
The carbon atom has four unpaired electrons in its outer shell so it can form four covalent bonds. The nitrogen has three unpaired electrons in its outer shell so it can form three covalent bonds and finally, oxygen has two unpaired electrons in its outer shell so it can form two covalent bonds.
In methane, one carbon atom forms four covalent bonds with four other hydrogen atoms
Ammonia NH3
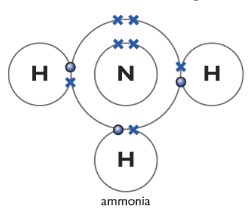
One nitrogen atom forms three covalent bonds with three other hydrogen atoms.
Water H2O
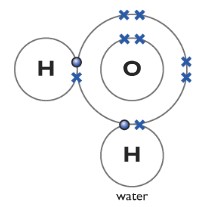
One oxygen atom forms two covalent bonds with two hydrogen atoms. Two lone pairs are left behind.
Oxygen (O2): In a molecule of oxygen, two oxygen atoms forms two covalent bonds with each other.
Carbon dioxide CO2
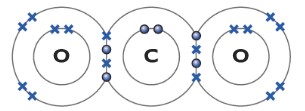
The carbon bonds with two oxygen atoms. The carbon atom forms two covalent bonding with each oxygen atom.
Nitrogen N2
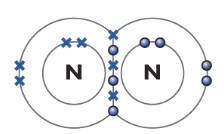
The nitrogen atom has three unpaired electrons in its outer shell, so it forms three covalent bonds with the other nitrogen atom.
Ethane C2H6
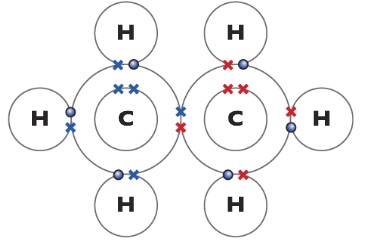
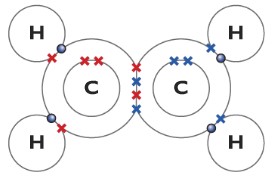
Ethene(C2H4)
Ionic Bonding or Electrovalent Bonding:
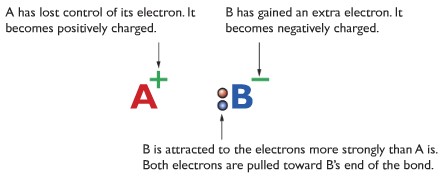
Ionic bonding is formed by gain or loss of electrons. The Metal atom loses electrons to form cations, and another non-metal atoma gains electrons to form anions. There is high electrostatic attraction between the cations and the anions.
ions are charged particles
cations are positive ions
anions are negative ions
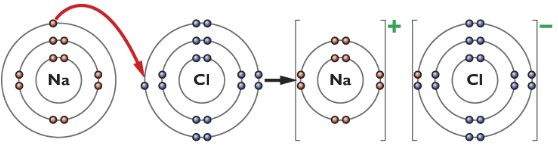
The dot-cross diagram of the bonding between sodium and chlorine
Another way to represent the bonding by writing down the electronic configuration
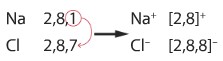
Explain in terms of electrons, what happens when sodium and chlorine reacts
The sodium atom loses an outer shell electron to form a Na+ ion and a chlorine atom gains that one electron to form a Cl– ion, there is an attraction between the Na+ and Cl– ions.
Showing the ionic bonding between magnesium and oxygen by writing electronic configuration:
Explain in terms of electron transfer what happens when magnesium and oxygen reacts
Ans: One magnesium atom loses it two outer shell electrons to form a Mg2+ ion and an oxygen atom gains those two electrons to form an O2- ion. There is an attraction between the Mg2+ and the O2- ions.
Showing the ionic bonding between calcium and chlorine by writing electronic configurations:
Explain in terms of electron transfer what happens when calcium and chlorine reacts:
One calcium atom loses its two outer electrons to form a Ca2+ ion, two chlorine atoms gains one electron each to form two Cl– ion. Now there will attraction between the oppositely charged ions.
Showing the ionic bonding between potassium and oxygen by writing electronic configurations:
Explain in terms of electron transfer what happens when potassium and oxygen reacts:
Two potassium atoms lose one electron each to form two K+ ions and one oxygen atom gains those two electrons to form an O2-. Now there will attraction between the oppositely charged ions.
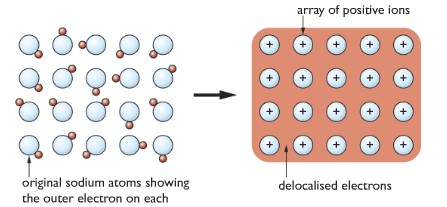
Metallic Bonding: The metal atoms lose their valence electrons to form cations and a sea of negatively charged electrons around them. Delocalized electrons attract each other.
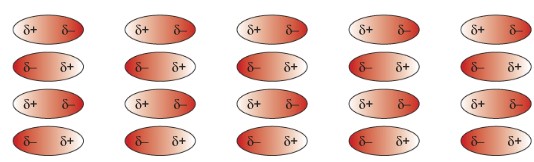
Intermolecular Forces: Weak forces between molecules. It easily overcomes with low energy.
Conclusion
So after reading this blog, now you have a clear idea about ionic bonding, covalent bonding, metallic bonding and also the inter-molecular forces. The concepts I have discussed in this blog is extremely important as the rest of the following chapters builds on these. It would be better you also refer to other chemistry sources if there are some terms if you are having difficulty in understanding.

Pingback: Preparation of salts and solubility of salts - IGCSE And IAL Chemistry
I think that whenever you try to show examples about covalent bonds you mention “Some Examples of covalent compounds”. Since the “compound” definition refers to a bonded atoms from different elements, I don’t think you should include diatomic molecules since are covalent bonds but the same element.