Enthalpy change is one of the most important concepts in chemistry. It does not matter what level of chemistry you are learning, your concepts of enthalpy change must be clear. In this blog, I am going to discuss in-depth the important concepts of enthalpy changes. After going through this blog, you will have a clear understanding of the following concepts:
What is Enthlpy ?
I am going to try to explain this concept very simple, avoiding all the scientific mumbo jumbo. In simple terms, enthalpy is the energy content which is within a substance. Or the internal energy stored within a substance. For example, an apple has some energy/enthalpy stored in it. Similarly, petrol also has enthalpy or energy content. The symbol of Enthalpy is H. But in this blog, we are more concerned about the change in enthalpy (ΔH), rather than
Introduction to Enthalpy Change
and their types
Have you ever been near to burning petrol or gasoline? If you have ever been, you must have noticed that it releases heat. But ever wondered why? Because when gasoline or petrol burns, it is reacting with the oxygen in the air. Below is a simple word equation of the reaction between petrol and oxygen.
Petrol + Oxygen → Carbon dioxide + Water
Here petrol and oxygen are reactants, they react with each other. While carbon dioxide and water are the products. The reactants have a combined enthalpy value. When the reaction takes place and products are formed, they have a new combined enthalpy value. In this case, the enthalpy of products produced is less than the enthalpy of the reactants. But what happened to the enthalpy which decreased? That is simply released to the surroundings, causing the temperature of the surroundings to increase. The decrease or increase of the enthalpy which occurs during a reaction, we know as the enthalpy change(ΔH). The unit of enthalpy change is Kilojoule per mole (KJ mol-1).
If you want to learn more advanced concepts of Enthalpy Changes, then you will find this book “Determination of the enthalpy changes of a chemical reaction using DTA: A new way to find out enthalpy measurement with DTA” on Amazon very useful.
Different types of Enthalpy Changes
There are two types of Enthalpy changes, they are:
- Exothermic
- Endothermic
Endothermic vs Exothermic reactions
Exothermic Reaction: When a reaction takes place and it causes the surroundings to heat up or cause the temperature of the surroundings to increase. That reaction is an exothermic reaction. In this type of enthalpy change, the enthalpy of the products is less than the enthalpy of the reactants. The value of the enthalpy change is negative (ΔH = -ve). The reaction between petrol and oxygen which I have mentioned is an example of an exothermic reaction. Because it releases heat or heats up the surrounding.
Endothermic Reaction: On the other hand when a reaction cools up the surroundings or decreases the temperature of the surroundings is an endothermic reaction. In this type of reaction, the enthalpy of the products produced is more than the enthalpy of the reactants. The value of the enthalpy change is positive(ΔH = +ve).
Bond breaking vs Bond making
The process of breaking a bond requires energy, it is
The process of bond-forming releases energy, negative. Energy is released when a covalent is formed or ions of opposite charges meet. If you are confused about how bonding is formed between atoms, please have a look at this article.
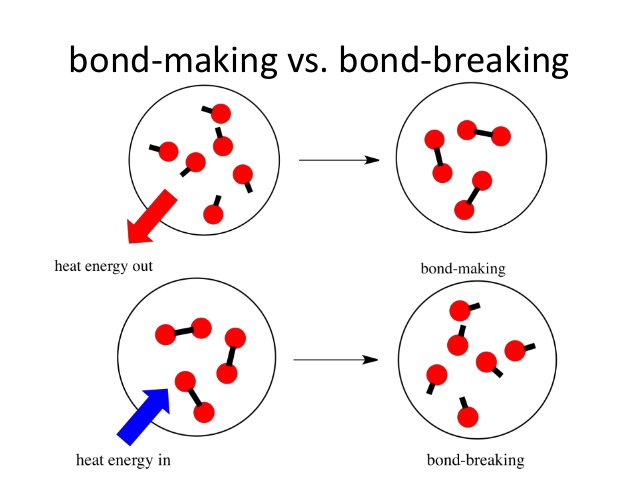
When a reaction takes place both the processes of bond breaking and bond making take place.
Firstly, all the bonds in the reactants are broken. This process requires energy, positive energy change.
Secondly, new bonds are formed when the products are formed. This process releases energy, negative energy change.
For example, have a look at the sample reaction below:
A+B → C + D
Firstly, all of the bonds in the reactants A and B are broken. This process requires or absorbs energy, positive change.
Secondly, new bonds in the products C and D are formed. This process releases energy, negative change.
The Mechanics of Exothermic and Endothermic Reactions
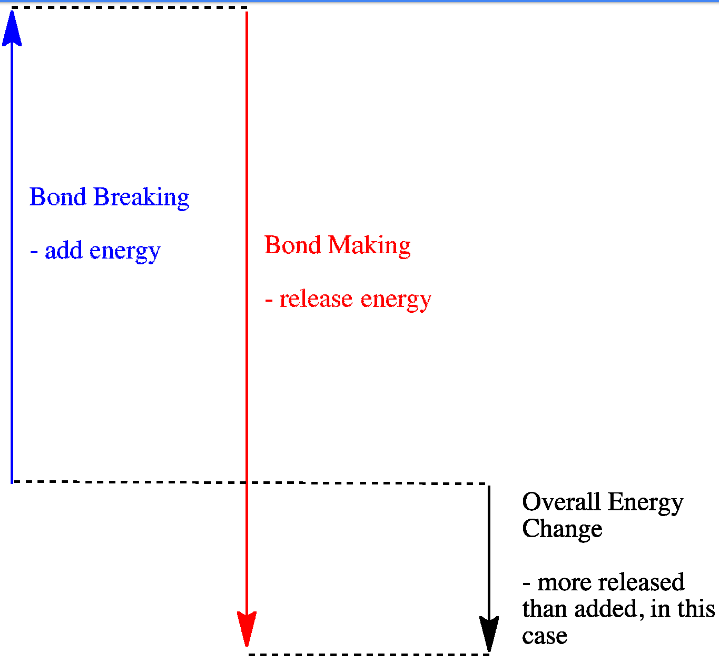
In an exothermic reaction, the energy released during the formation of bonds is more than the energy absorbed during the breaking of bonds. The extra heat energy released is known as ΔH (enthalpy change), which is negative.
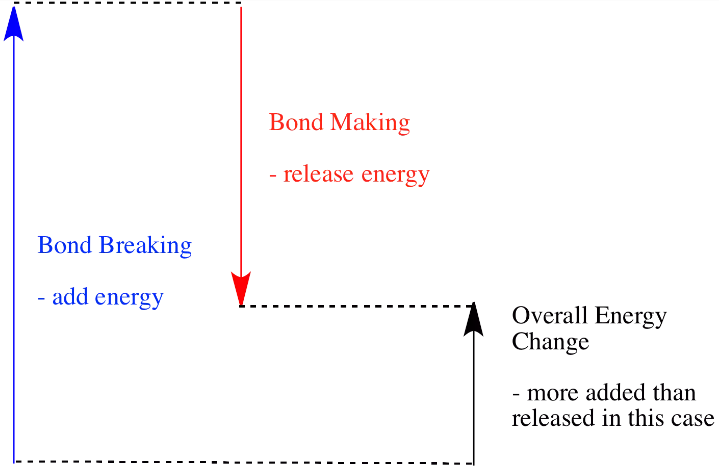
In an endothermic reaction, the energy absorbed or required during the breaking of bonds is more than the energy released during the formation of bonds. The extra heat energy required is absorbed from the surroundings, the enthalpy change is positive.
For example the combustion of methane (reaction of methane with oxygen)
CH4 (g) + 2O2(g) →CO2(g) + 2H2O(g) ΔH= -ve
It means that more energy was released during the formation of bonds in the products CO2 and H2O than the energy absorbed during the breaking of bonds in the reactants CH4 and O2.
Usually, exothermic reactions are more common than endothermic reactions.
Examples of exothermic reactions:
Neutralization reaction between acid and alkali.
Examples of endothermic reaction:
Decomposition of calcium carbonate:
CaCO3 →CaO(s) + CO2
Photosynthesis: A process in which glucose and oxygen are made from carbon dioxide, water using energy from the sun is also endothermic.
Unit of enthalpy change → KJ/mol
What does the unit say?
The unit
For example:
CH4 (g) + 2O2 (g) → CO2 (g) + 2H2O (g)
ΔH= -220 KJmol-1 (made up value for explaining)
It means that for per mole of methane reacting 220 KJ of heat energy will be released to the surroundings.
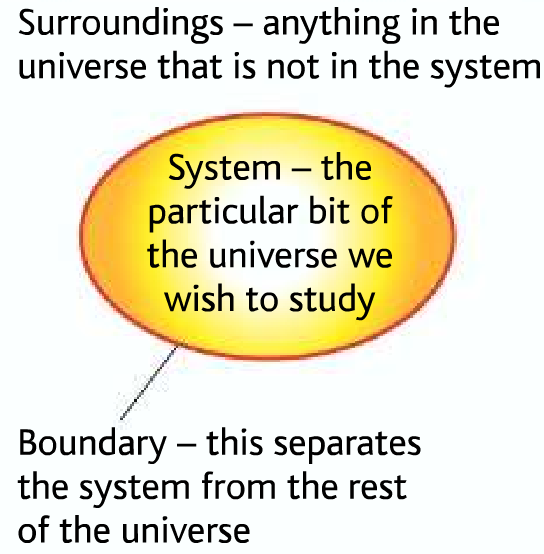
System and surroundings
System → The reactants and products
Surroundings→ The air, apparatus, solution, you and everything else.
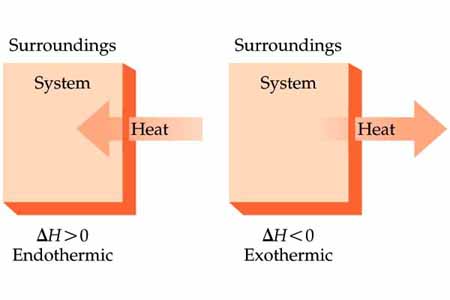
In an exothermic reaction, the excess heat is released from the system to the surroundings.
In an endothermic reaction, the excess energy required by the system is taken from the surroundings.
Example: HCl(aq) + NaOH(aq) → NaCl(aq) + H2O(l) ΔH= -100 KJmol-1
It means that for per mol of reactant reacting, 100 KJ of energy is released from the system to the surroundings.
System→ NaOH, HCl, NaCl, and H2O
1st Law of thermodynamics: Energy can neither be created nor be destroyed, it can only be transferred from one form to another.
It means that the energy content in the universe is constant.
D] Enthalpy: The energy content of a system held at constant temperature and pressure.
Symbol→H
Enthalpy cannot be measured, but enthalpy change can be as it is accompanied by a temperature change from which the energy released or absorbed can be calculated.
Symbol→ ΔH
When a system reacts at constant temperature and pressure. Gives or takes in heat energy, enthalpy change occurs.
ΔH = Hproducts – Hreactants
When petrol is burned enthalpy change is what really matters.
ENTHALPY LEVEL DIAGRAMS:
For Exothermic reaction, ΔH is negative, which means the system loses energy to the surroundings. The products formed will have less energy than the reactants.
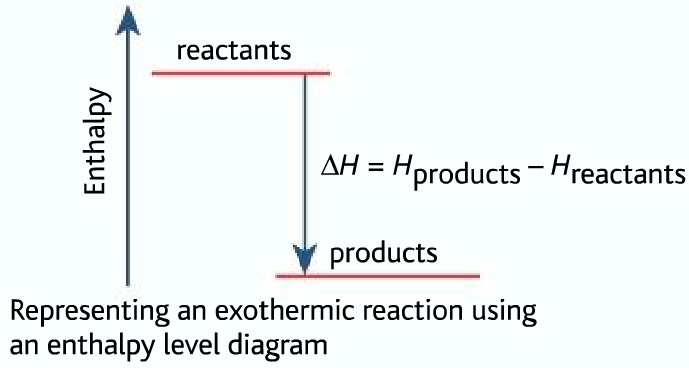
Hproducts <Hreactants
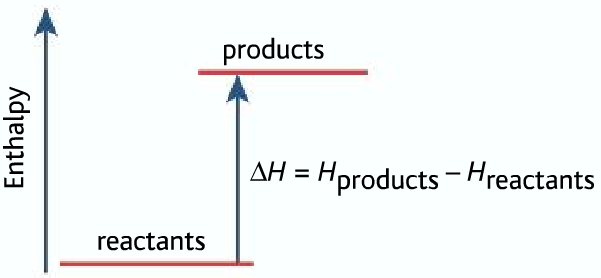
For endothermic reaction ΔH is positive, it means that the system gains energy from the surroundings and products are formed. The products formed will have more energy than the reactants.
Using exothermic and endothermic reactions:
Exothermic
- Used in heat packs, heat packs used by sports people to relax their muscles.
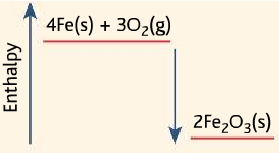
- The heat pack uses the reaction of oxidation of iron (iron and oxygen reacting), which is exothermic.
4Fe (s) + 3O2(g) → 2Fe2O3
- A damp mixture of iron filings with salt and charcoal in a perforated bag. When the seal is broken, oxygen enters reacts with iron to release heat.

- The reaction is speeded up by the presence of salt and charcoal.
Endothermic reaction:
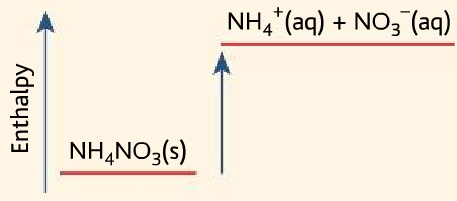
- Used in cold packs, cold packs are used to treat injuries.
- It uses the endothermic reaction between ammonium nitrate and water.
NH4NO3 (s) →NH4+ (aq) + NO3– (aq)
Conclusion
Thank you for reading this blog. I hope now your concepts about enthalpy change is absolutely clear. Now it would be much easier for you to face more advanced topics in Chemistry.
Also, check out the other chemistry blogs I have written. I am sure you will find something useful. Good Luck!