The Mass Spectrometer is a device used to identify different elements and compounds in a substance by finding out their molecular mass. It uses the concept of mass: charge (m:z) ratio to find the molecular formula.
There are six steps in the mass spectrometer, they are:
- Vaporization
- Ionization
- Acceleration
- Velocity selector
- Deflection
- Detection
We are going to have a closer look at these six steps and learn how they work together. In this IAL chemistry mass spectrometer note, I am going to discuss how it works and the different uses of the mass spectrometer. I am going to remain within the boundaries of IAL or A-level chemistry, discussing only the topics included in the syllabus.
Relative Isotopic Mass VS Relative Atomic Mass
Before we dive into how a Mass Spectrometer works, firstly let’s have a clear understanding of Relative Isotopic Mass and Relative Atomic Mass.
Relative Isotopic Mass
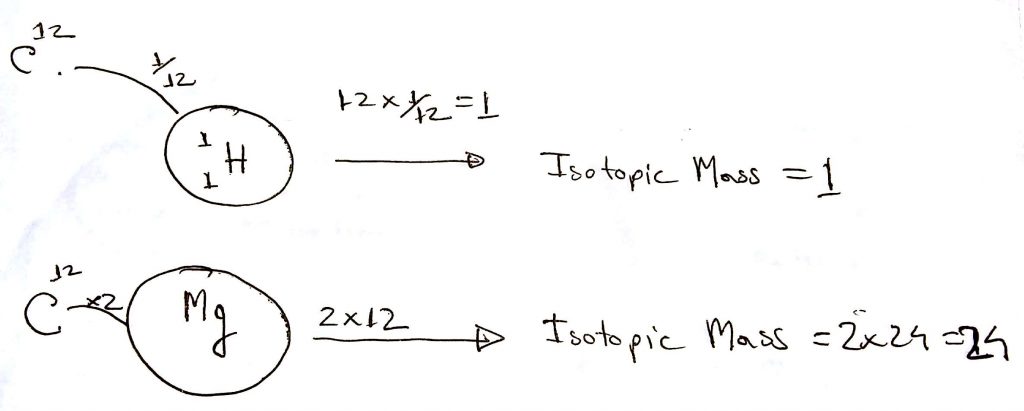
Atoms are very small. You cannot just take a measuring scale and measure its mass. To get an idea about the mass of atoms, scientists chose another way. They picked the most common isotope of Carbon which contains 6 protons and 6 neutrons. Then they assigned its mass to 12 atomic units. This carbon is most commonly known as Carbon-12.
Scientists took this Carbon-12 isotope as a standard than found out the relative isotopic mass of the rest of the elements by comparing this to the Carbon-12 isotope.
For example, the mass of a Hydrogen isotope got a mass of 1/12 of a Carbon-12 isotope. Hence its relative isotopic mass is 1 atomic unit.
Another example, the mass of a Magnesium isotope is twice that of Carbon-12, hence its relative isotopic mass is 12 times 2, which is 24.
Please have a look at the diagram below to have a better understanding of Relative Isotopic Mass.
Relative Atomic Mass
On the other hand, the Relative Atomic Mass of an element is the weighted average of all the mass of isotopes on that element, relative to their abundance. We will have a closer look at how we can find the relative atomic mass from the mass spectrometer reading. Later on in this blog post.
Relative Formula Mass vs Relative Molecular Mass
We also need to have a clear understanding of the terms Relative Formula Mass and Relative Molecular mass. Relative Formula Mass is the sum of all the Relative Atomic masses of atoms present in a compound. It is also denoted by the symbol Mr.
For example, NaCl (sodium chloride) is a compound. The relative atomic mass of sodium(Na) is 23. The relative atomic mass of chlorine is 35.5. Hence the relative formula mass of NaCl is.
Mr (NaCl) = 23 + 35.5 = 58.5
So what does relative molecular mass mean? It is exactly the same as the relative formula mass. But the term relative molecular mass is used when a compound is made out of molecules.
For example, CO2(Cardon dioxide) gas is made out of molecules. So for this, we will use the term relative molecular mass. The symbol will be the same.
Mr (CO2) = 12 + (16 x 2) = 44
What is a mass spectrometer
The mass spectrometer is a device that accurately detects which elements are present in a compound. It does that by finding out their relative atomic or relative formula mass. It uses the concept of mass: charge (m:z) concept. We will learn more about the mass: charge ratio concept in this blog. So no need to worry.
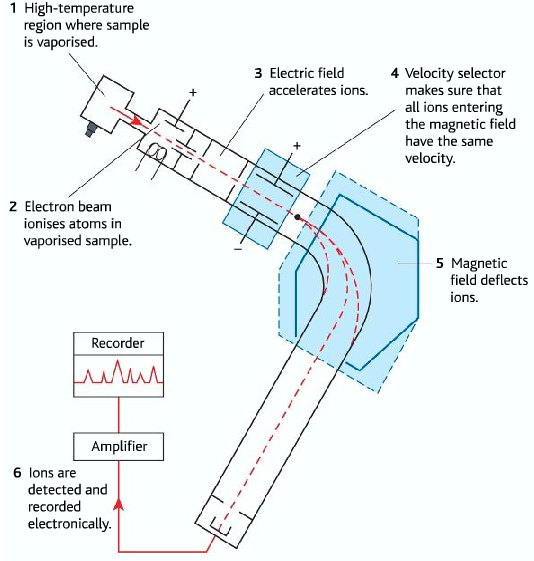
Different steps of the mass spectrometer
| Step Name | Function the step | |
| 1 | Vaporization | A high-temperature region where the sample is vaporized |
| 2 | Ionization | Electron beam ionizes atoms in the vaporized sample |
| 3 | Acceleration | the electric field accelerates ions |
| 4 | Velocity selector | The velocity selector makes sure that all ions entering the magnetic field have the same velocity. |
| 5 | Deflection | The magnetic field deflects ions |
| 6 | Detection | ions are detected and recorded electronically |
1. Vaporization
In the vaporization step, the sample is inserted into a very high-temperature region. This high temperature causes the sample to turn into gas. Whether the sample is solid or liquid it turns into gas. When the sample turns into gas the particles become free to move around.
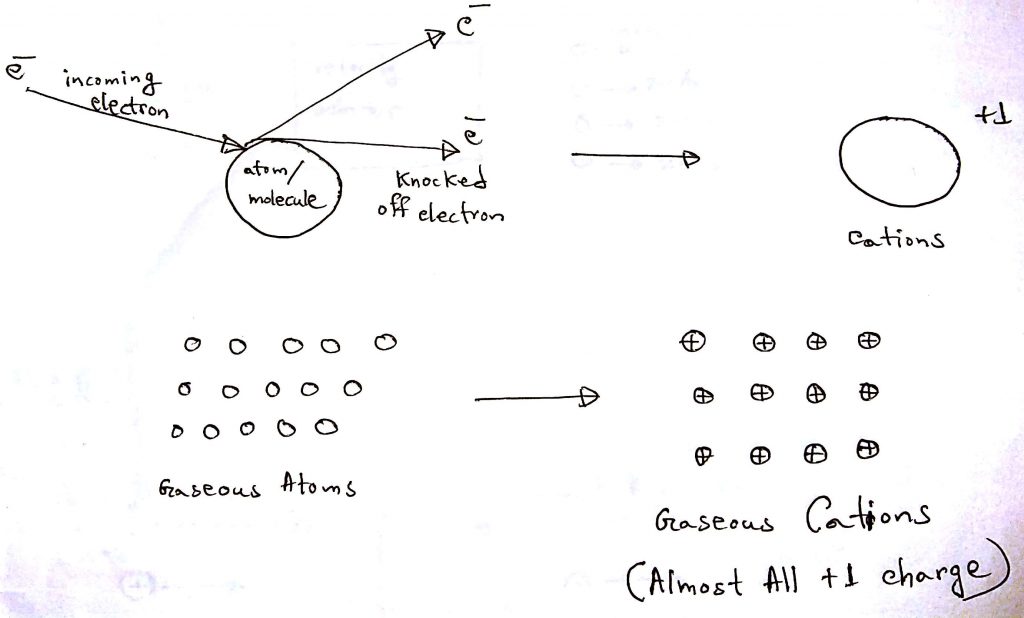
2. Ionization
In the ionization step, the gaseous sample is bombarded with high-energy electrons. When the electrons hit the particles of the gaseous sample, it knocks off electrons from the particles. The electrons usually knock off one electron because they do not have the energy to knock off multiple electrons. In the end, the particles end up being mostly +1 cations. After losing the electrons the mass of the particles are almost unchanged because electrons have negligible mass.
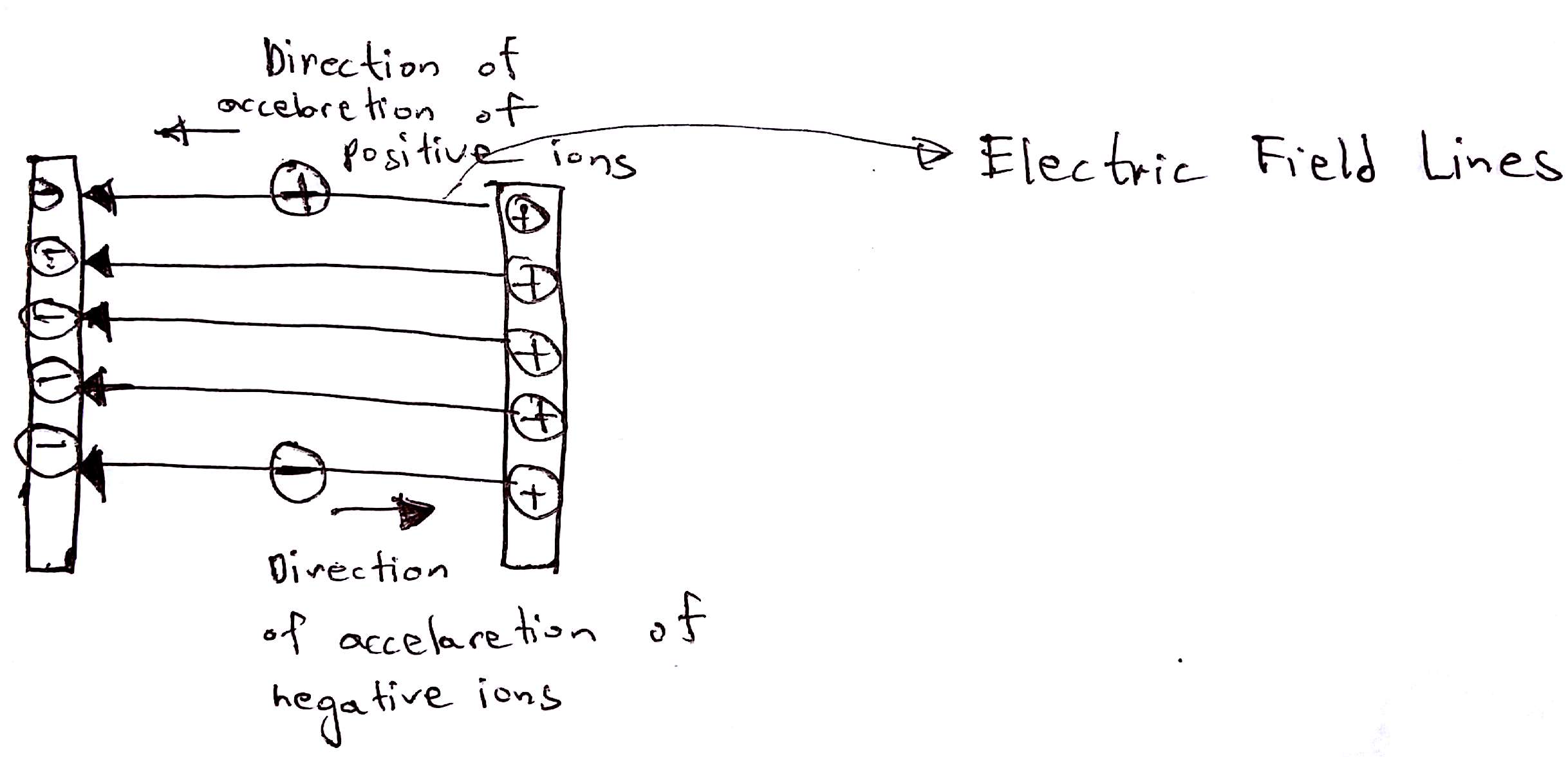
3. Acceleration
In this step, the ions are accelerated by an electric field. Charged particles feel the force when they are introduced into an electric field. Positive ions feel a force in the direction of electric field lines whilst the negative ions will feel the force in the opposite direction that of the field lines. When an object feels force it accelerates. Hence the ions speeds ups achieve a high speed.
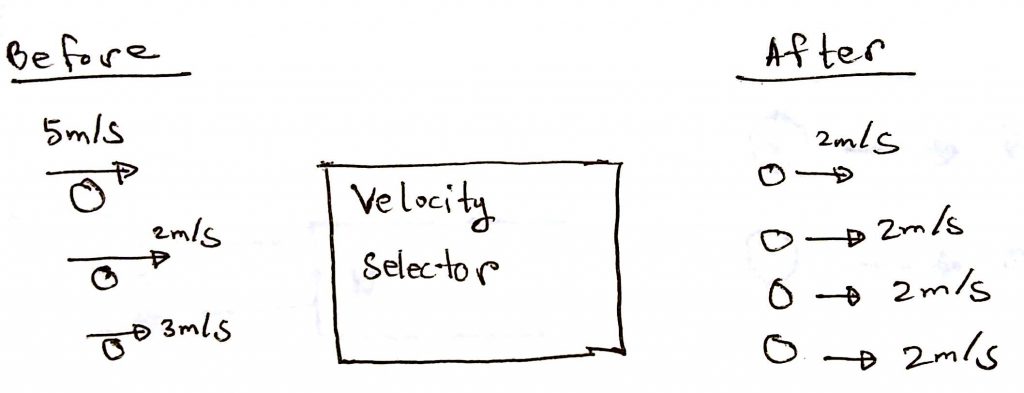
4. Ions achieve uniform velocity
In this area, the ions are introduced in an area called the velocity selector. This step makes all the ions have the same velocity. This step is very important for the next step. Below is a simple diagram to make you understand. The velocities are made up, not real.
5. Defelection
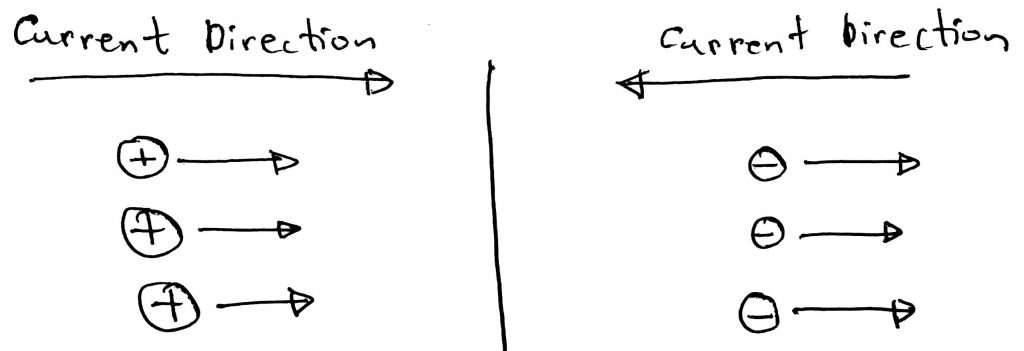
Firstly let’s understand what the flow of charged particles means. The flow of ions means the flow of current. The direction of the current depends on whether the ions are positive or negative. If positive ions move in a certain direction then the current is in that direction. If negative ions move in a direction then the current is in the opposite direction. Please have a look at the diagram below to have a better understanding.
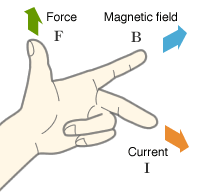
According to Flemings Left Hand Rule when a current passes through a magnetic field it feels a deflection. As the moving charged particles mean current, they will feel the force and will be deflected from their straight path.
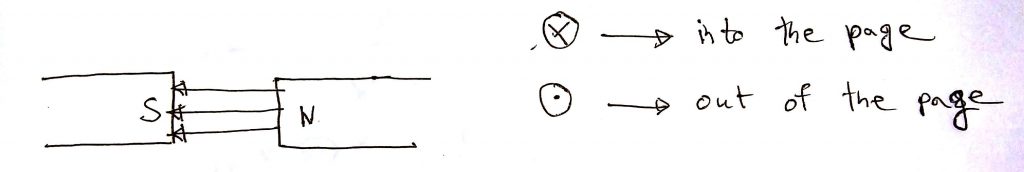
In the diagram below the magnetic field is going into the page. The cations are moving down, hence the current is also downward. According to Fleming’s left-hand rule, the cations will be deflected rightwards.
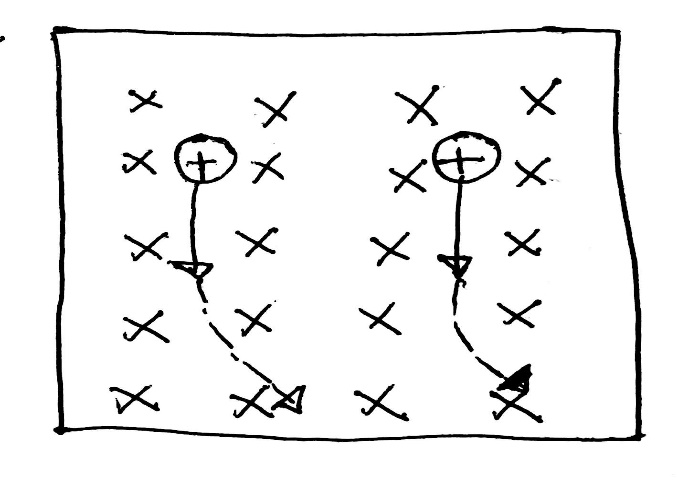
Given that the cations have the same charge, the heavier cations will deflect less than the lighters ones.
Given that two cations have the same mass. But one has got a higher charge, the higher charge ion will deflect more.
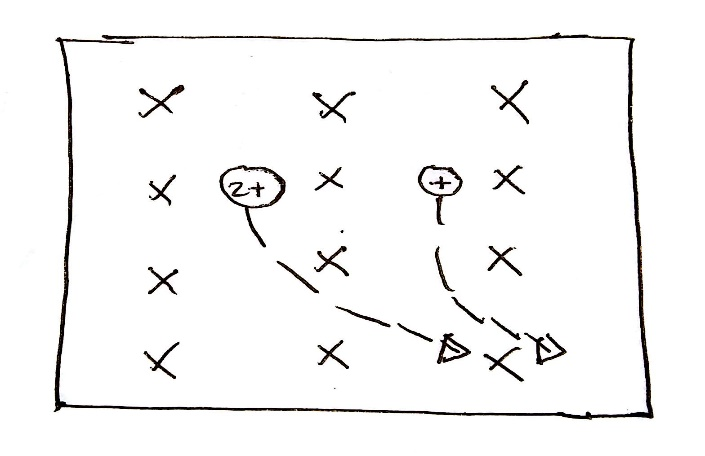
Finally coming to the main process, assuming all the ions are of +1 charge after passing the ionization step. When they move through the magnetic field, they are all deflected. Heavier ions are deflected less than lighter ions. As it can be seen, this part of the mass spectrometer is bent. Only the perfect deflection will cause the path of an ion of a specific mass to bent and be detected by the mass spectrometer.
What is the mass: charge (m:z) ratio?
Understanding with an example:
Suppose, given
Na+ ion
Mass=23
Charge= +1
Therefore, mass:charge (m:z) ratio = = +23
- The lower the m:z ratio, the higher the deflection.
- An ion of specific m:z ratio will need a specific magnetic field strength for the perfect deflection, so the ion can reach the detector.
6. Detection
As we know that an ion of a specific m:z ratio will need a specific magnetic field for it to be detected.
Suppose (this example is just for understanding, the value of the magnetic field is a randomly made up value)
Na+ m:z ratio = +23
Suppose it needs 4T of magnetic field for perfect deflection and to be detected.
Imagine that we put an unknown sample in the mass spectrometer. Then we have set the magnetic field to 4T. Then we start to have detection. It means we are detecting an ion of mass: charge ratio of +23, which is Na+ !!
The mass spectrometer varies the magnetic field and calculates the number of ions detected for the expected m:z ratio, at a specific magnetic field. The output is the relative abundances of the ions of each mass detected.
As I have said before, it is unlikely for a particle to lose more than one electron during ionization. So assume all ions are of +1 charge.
Again using sodium as an example:
Mass=23
Charge=+1
Mass:Charge ratio = 23/+1 = +23
- The value of the mass:charge ratio is the mass of the particle. Ignoring the positive sign.
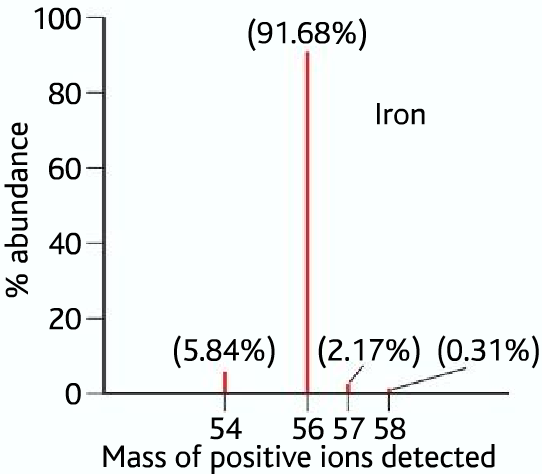
Here is a sample mass spectrum (data) of iron, as we know that iron contains different isotopes which have different masses, which means different m:z ratios. So there will be peaks for each different isotope, stating their relative abundances.
Interpreting the mass spectrometer data
Finding the R.A.M using mass spectrometer data
First Example: Mass Spectrum of Sodium
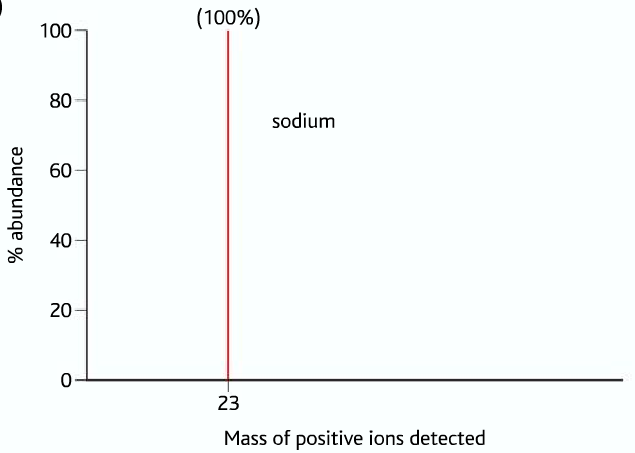
In the mass spectrum of sodium, there is only one peak at mass 23, which is 100%. It means that sodium has only one isotope, whose mass is 23. Therefore the R.A.M of sodium is 23, Ar(Na) = 23 .
Second Example: mass spectrum of iron
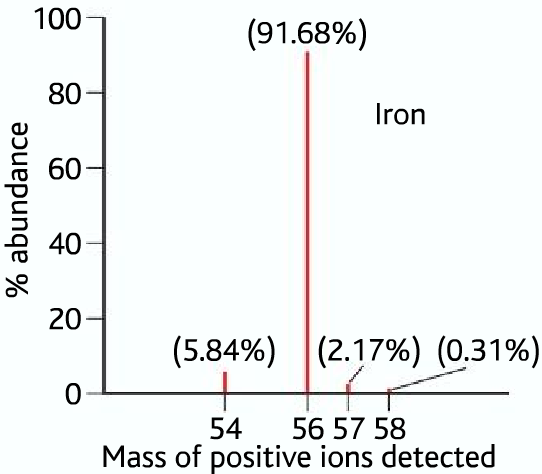
From the above data, we can see that iron has four isotopes of masses 54, 56,57, and 58. Their relative abundances are as follows: Iron-54 5.84%, Iron-56 91.68 %, Iron-57 2.17%, and Iron-58 0.31%
Therefor R.A.M of iron or Ar (Fe) =
(54 x 5.84%) + (56 x 91.68%) + (57 x 2.17%) + (58 x 0.31%) = 55.91
Third Example: mass spectrum of chlorine
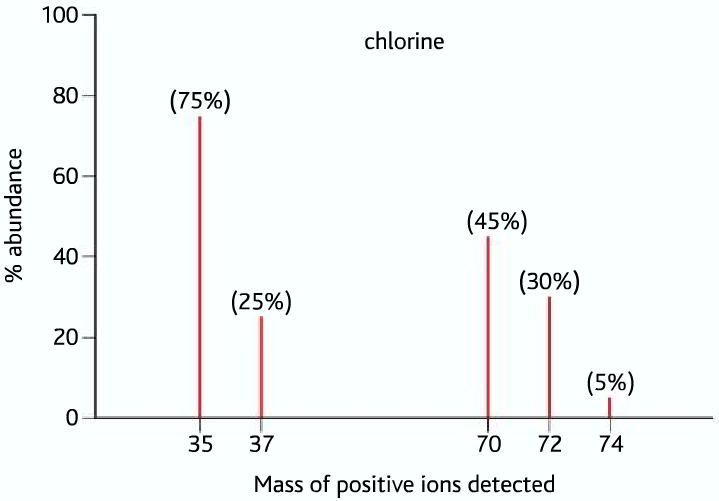
From the above mass spectrum of chlorine, we can see peaks at 35 and 37 which are the isotopes of chlorine, Chlorine-35 has a 75% relative abundance, while chlorine-37 has a 25% relative abundance. But what do the peaks at 70,72,74 represent? They are the molecular ion peaks of chlorine, during ionization, the choline molecule breaks to form 35Cl+ and 37Cl+ ions, but it doesn’t mean that all of the chlorine molecules form these. Some of the molecules are turned into cations as a whole, these three peaks represent these molecular ions as a different combination of 35Cl and 37Cl.
Peak 70 → (35Cl-35Cl)+
Peak 72 → (35Cl-37Cl)+
Peak 74 → (37Cl-37Cl)+
Practice
From the above data, you can see that Chorine has two isotopes, Chlorine-35’s relative abundance is 75% and Chlorine-37’s relative abundance is 25%. Find the R.A.M of Chlorine in the similar way you found that of iron.
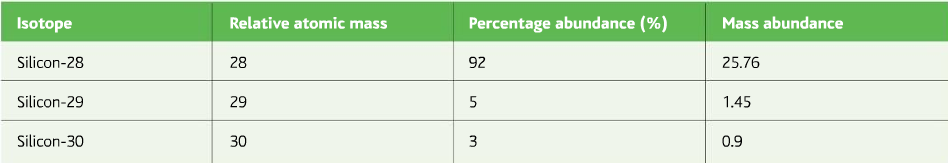
Now find the R.A.M of Silicon from the data given in the table below:
Different uses of the mass spectrometer
Mass Spectrometer in the pharmaceutical industry
The mass spectrometer is very useful in the pharmaceutical industry. It is very sensitive, small samples are measured and analyzed accurately. It can differentiate between similar compounds, such as isomers (molecules with the same molecular formula but different structural formula). It is important because our bodies react differently to different types of isomers differently.
What is High-Performance Liquid Chromatography (HPLC)?
Answer: The concept and purpose are the same as the paper chromatography.
Concept: Different substances in a compound have different solubility in a given solvent. So when the solvent moves, they move along at a separate rate and get separated.
Purpose: To separate the different substances present in a compound.
In paper chromatography, the paper is the main medium through which solvent moves. The solvent is usually water.
But in HPLC, the medium is SiO2 (Silica), which is packed in a steel tube. The chemicals to be detected are packed at one end. The solvent (depending on which one to use may vary) Is pumped from one end at high pressure. Which separates the substances in a compound. Then each of the separated substances is analyzed using the mass spectrometer for detailed analysis.
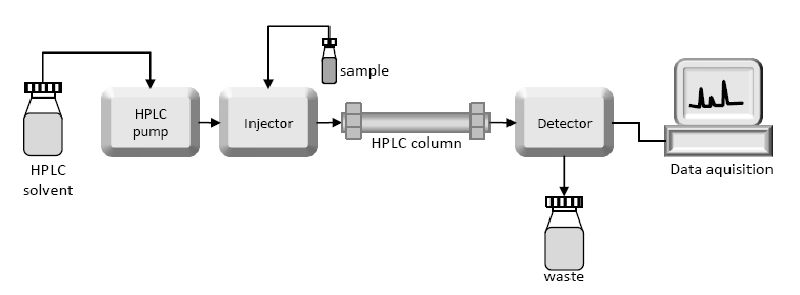
Using the mass spectrometers the relative formula mass of a compound can be figured out and the compound can be detected.
Drug Testing- detecting the cheats
Anabolic steroid is a drug that helps to build bigger muscles. It is usually the artificial form of the male hormone testosterone. In the human body, there is are two hormones testosterone and epitestosterone. The testosterone: epitestosterone (T: E) ratio is never greater than 4:1. A person taking anabolic steroids has higher ratios of about 6:1. The mass spectrometer detects the T: E ratio in the body, these hormones are excreted out in the urine. In synthetic testosterone, the 12C:13C ratio is different from that of the natural.
Radioactive Dating
Radioactive Elements: Some elements are radioactive, and they undergo radioactive decay. Radioactive decay is a process in which the unstable nucleus breaks apart to become more stable, and emits radiation.
There are three types of radioactive decay: alpha, beta, and gamma:
- Alpha particles consist of two protons and two neutrons 24He.
- Beta particles are high-energy electrons.
- Gamma rays are electromagnetic radiation.
During radioactive decay, the radioactive isotope becomes a different isotope, often a different element. Because its proton number changes. The radioactive process is spontaneous and random: meaning that it occurs naturally and random meaning that it can’t be said which atom will break up. Any atom can break up. It is not influenced by any external conditions such as temperature and pressure. But the half-life of a radioactive isotope is constant.
Half-Life: The time taken for half of the radioactive isotope to decay in a given radioactive sample. It can be from seconds to millions or billions of years. Depending on the isotope.
For example:
The half-life of Radon-222 is 3.82 days. It is an alpha emitter, that decays to form polonium-218
Explaining using number of particles:
Suppose we start with 1000 particles of Radon-222. After 3.82 days it would halve to 500 Radon-222 particles. Then again after 3.82 days, it would halve again, to 250 particles. This goes on.
Explaining using mass:
Suppose we begin with 1g of Radon-222. After 3.82 days it would halve to 0.5g. Then again after 3.82 days, it would halve again to 0.25g. This goes on.
Showing Example below beginning with N atoms, the half-life is t1/2
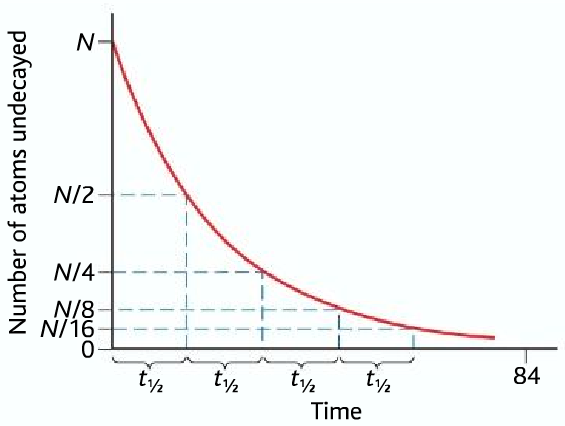
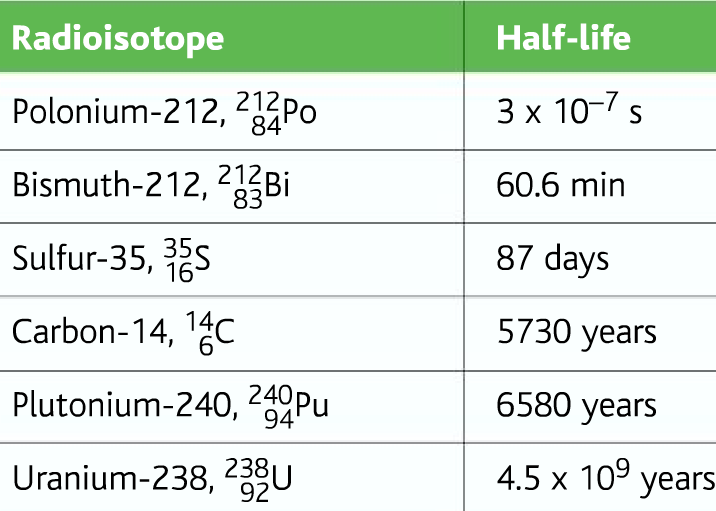
Sample Half-lives of different radioactive elements:
Radioactive material uranium is used in the nuclear power industry and nuclear weapons.
Radioactive Dating: This method is used to find the age of ancient remains. The radioactive carbon-14 isotope is used in this method. The CO2 in the atmosphere has 0.00000000010% carbon-14, rest majority is carbon-13 or carbon-12. Living plants take in CO2 and which means they also take in Carbon-14. So do the animals when they eat plants. So the above percentage of carbon-14 is maintained. But when the animal or plant dies this percentage starts to drop. We know the half-life of carbon-14 is 5730 years. So from the percentage drop, we can calculate the age. The mass spectrometer finds the amount of carbon-14 in a material.
Conclusion
So in this blog post, we have learned the different steps of the mass spectrometer. How each step works. And finally some important uses of the mass spectrometer.


Hey There. I found your blog using msn. This
is a very well written article. I will be sure to bookmark it
and return to read more of your useful info. Thanks for the post.
I will certainly comeback.