The Periodic Table
The periodic table which also used to be known as the mendeleev table lists down all the elements which have been discovered till now. In the modern periodic table, the vertical columns are known as groups whereas the horizontal rows are known as periods. The elements are arranged in order of increasing atomic number. As you go from left to right, the proton number increases by one.
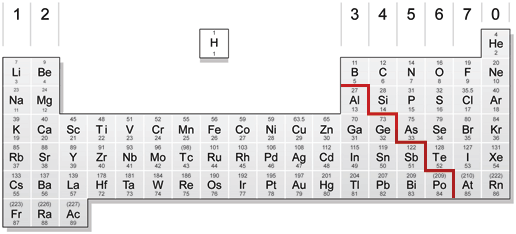
You can see a red stair, on the left side of the stair all the elements are metals, all the elements on the right side of the stair are non-metals.
- All the elements in the same group have the same number of electrons in the outer shell
- All the elements in the same period have the same number of shells
To learn more about the periodic table in a fun way, you can read this book “The Elements Book: A Visual Encyclopedia of the Periodic Table” from Amazon.
Some differences between metals and non-metal
Metals
- Metals have high densities, melting and boiling points
- The are shiny
- They are Malleable, ductile
- Good conductor of heat and electricity
- They form cations or positive ions
- It forms oxides which are basic, meaning they produce alkaline solution when dissolved in water
Non-metals
- Most non-metals have low melting and boiling points (except carbon and silicon due to giant covalent bonding)
- They are Brittle, means they break easily with little force
- They are poor conductors of heat and electricity (except graphite and silicon)
- Most non-metals form negative ions or cations
- Its oxides are acidic, meaning they dissolve in water to form an acidic solution. For example, hydrogen chloride gas dissolves in water to form hydrochloric acid.
Group 0 or Noble Gases
- All of them have full outermost shell
- They exist as monatomic(single atoms instead of compounds or molecules). They are in gaseous state because of their inability to form covalent bonds due to their full outermost shell
- Melting and boiling points of noble gases increase down the group(as the size of atoms increases) because there is more attraction between atoms.
- Inert, due to full outermost shell.
Characteristics of Group 1 elements or Alkali Metals
- They are very Soft, have lower density than other metals, also lower melting and boiling points
- They all are highly reactive
- Most of the reactive ones must be stored under oil, to prevent them from reacting with air(oxygen in the ait) or with water vapor.
- Reactivity of group one metal increases down the group
- They form +1 charged ions when forms compounds
- All of the group 1 compounds are white, they all dissolve to form a colorless solution
Why does the reactivity of group one metals increase down the group?
Because down the group the number of shells increases, therefore, the outer shell electrons are further away from the nucleus, they feel a less attractive force towards the nucleus, hence can be more easily removed.
How Group 1 metals react with water
All of the group 1 metals react vigorously with water, they all react with water to produce metal hydroxide and hydrogen gas.
How sodium reacts with water: Sodium reacts vigorously with water, it floats on the surface of the water, as its density is less than water. There is a vigorous effervescence of hydrogen gas, hydrogen gas is released rapidly which causes sodium to dart on the surface of the water. The reaction is highly exothermic, releases tons of heat
2Na(s) + 2H2O(l)→ 2NaOH(aq) + H2(g)
How Lithium reacts with water Lithium reacts similarly to sodium reacts with water, but reacts a little bit slower than sodium, as its reactivity is less than sodium.
How potassium reacts with water is similar observation in the case of potassium, but reacts much more vigorously, the potassium metal even catches fire and burns with a lilac flame.
Rubidium and Caesium with water these metals react so vigorously with water, that it is dangerous to carry out their reaction with water and they are hardly used.
Group 7 also known as Halogens

- Reactivity of halogens decreases down the group
- Melting and boiling point of halogens increase down the group
| Halogen | State | Color |
| F2 (Fluorine) | gaseous | yellowish |
| Cl2 (Chlorine) | gaseous | green gas |
| Br2 (Bromine) | liquid | Dark red liquid, red-brown vapor on heating |
| I2 (Iodine) | solid | The dark gray solid, purple vapor released on heating |
The reaction of halogens with hydrogen
When halogens react with hydrogen they form hydrogen halides, hydrogen halides dissolve in water to form an acidic solution.
Reactivity or the oxidizing power of halogens decreases down the group because the number of shells increases also the incoming electron will be further away from the nucleus, hence feeling less attractive force toward the nucleus.
More reactive halogens are able to displace the less reactive ones but not the other way round
H2 (g) + Br2 (g) → 2HBr (g)
| Halogen | Reaction with hydrogen |
| F2 (Fluorine) | Violent explosion occurs, even if the reaction is carried out in the cold and dark |
| Cl2 (Chlorine) | Violent explosion occurs given that the mixture is exposed to flame or sunlight. |
| Br2 (Bromine) | Mild explosion if bromine vapor and hydrogen gas is mixed and exposed to flame |
| I2 (Iodine) | The partial reaction occurs if hydrogen and iodine are heated constantly. |
Transition Metals
- They all have Typical metallic properties such as high melting and boiling points, ductile, malleable etc
- They all are less reactive than group 1 and 2 metal due to this property they have various practical uses.
- Transition metals tend to form colorful compounds
- We mostly use transitional metals and their compounds as catalysts. For example Iron in the manufacture of ammonia, Vanadium(V)Oxide in the manufacture of sulfuric acid and also manganese(IV)oxide in the decomposition of hydrogen peroxide.


Hi,
I suggest you to create a full complete PDF file of the whole notes of all the chapters, with your own template. That way it would be easier for students as they can download the file and use it.
Thanks,
Hello Yasir, thank you so much for your valuable comment. I am extremely happy that you are finding my contents helpful. I will work on preparing PDF files for the chapters and make arrangements so that students will be able to download those PDF.