Why this the BEST Organic Chemistry note
If you are an IGCSE Chemistry student desperately looking for the perfect organic chemistry notes, then this is the one! Why? Because I have written this blog post specifically for IGCSE chemistry students. This blog only covers the organic chemistry topics which are included in the IGCSE chemistry syllabus. No more, no less.
I have got an A in IGCSE chemistry. Not only that, 15 out of 15 of my IGCSE chemistry students whom I have taught as a part-time job either got an A* or an A in chemistry. All of them aced in organic chemistry. I have emphasized the topics which appear frequently in IGCSE chemistry exams. I have explained everything to the point and in an easy-to-understand way, no-nonsense. After you finish this, you will definitely be more confident with organic chemistry.
Organic Chemistry – Study of Hydrocarbons & their derivatives
Organic Chemistry is the study of compounds based on hydrocarbons and their derivatives. This organic chemistry note for IGCSE chemistry will cover the three major homologous series Alkane, Alkene, and Alcohols. We will discuss their general formula, physical and chemical properties. I have covered all the organic chemistry basics with a complete breakdown. Please keep on reading and enjoy!
Hydrocarbon
A Hydrocarbon is a compound that is made of hydrogen and carbon only.
We are going to learn about four main homologous series; they are Alkane, Alkene, Alcohol, and carboxylic acid.
If you want to learn the basics of Hydrocarbons, then you can also have a look at my blog “Hydrocarbon Classification, Names, and General Formulas“.
Organic Chemistry is a very tricky topic. If you are absolutely new to organic chemistry and need a base knowledge, then check out this awesome book “Organic Chemistry I For Dummies (For Dummies (Lifestyle))” on Amazon.
Homologous series
The Homologous series is a family or group of hydrocarbons or derivatives having the same functional group, same general formula, and similar chemical properties. They also have a similar trend in physical properties.
Functional Group
The functional group is simply an atom or group of atoms attached to the organic compound which dictates its chemical property. Gives its own chemical property. Also in some cases, it gives rise to some unique physical properties too. Do not worry if you are still confused about what a functional group is. I have explained this concept with some examples in this blog, that will make everything clear.
General Formula
The General Formula is the formula to find out the molecular formula for an organic compound that is in a certain homologous series.
The general formula of a homologous series gives the formula which shows how many hydrogen or another kind of atoms will be there for a given number of carbon atoms.
For example, the General Formula of Alkane is
Simple differentiating of the four homologous series:
Alkane
Alkane is the simplest of all the homologous series. It only has simple C-H bonds throughout its whole structure. The General Formula of the alkane is
For example, Butane is an alkane with four carbon atoms. We can use the general formula of alkane to easily figure out its molecular formula.
The number of Carbon atoms is 4, hence n=4. Hence the molecular formula of Butane is C4H2(4)+2 = C4H6.
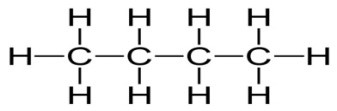
Alkene
Similar to Alkanes, alkenes also contain C-H bonds. But it also contains one C=C (carbon to carbon double bond) attached as the functional group, its general formula is CnH2n
The functional group of alkenes gives it some unique chemical properties. I will discuss that later on in this blog with more details.
For example, butene is an alkene with four carbon atoms. We can easily use the general formula of alkene to determine the molecular formula of butene.
Butene has four carbon atoms, hence n=4. Hence the molecular formula of butene is C4H2(4) = C4H8
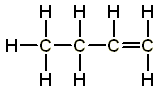
Alcohol
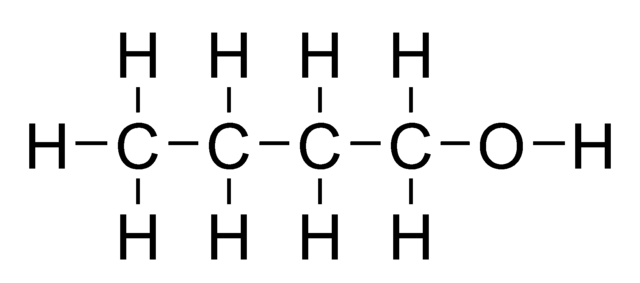
In Alcohol, there are also C-H bonds present. The –OH(Hydroxide) attached acts as the functional group, general formula CnH2n+2O.
Similar to alkanes and alkenes, we can also use the general formula to determine the molecular formula of specific alcohols.
For example, butanol is alcohol with four carbon atoms. For a more advanced blog post on alcohol, please view this blog.

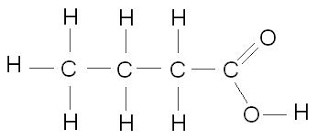
Carboxylic acid
C-H is present, but with –COOH bond attached as the functional group. General Formula CnH2nO2
For Example butanoic acid, a carboxylic acid with four carbon atoms. Please note, the carbon atom within the functional group is also taken into consideration.
Naming an organic compound
Naming an organic compound is one of the most important things to do. In IGCSE chemistry exams, they give the formula and ask you to name it. So read this section very carefully as I dive into details of how to name an organic compound.
The name of an organic compound indicates four things:
- The number of carbons atom(s) in the compound.
- If an alkyl group attached or not. If yes, then its name and position number.
- Indicating to which homologous series the organic compound belongs to and the position number of the functional group if any present.
The number of carbon atom(s) present is indicated by the prefix (the beginning part of the
The homologous series to which the compound belongs to is indicated by the suffix (end part).
The alkyl group position number and name is indicated before the prefix.
The position no. of the functional group is placed between the suffix and prefix.
So the name of an organic compound is in the following format:
m code Alkylname prefix-n-suffix
m- Position of the alkyl group
n- position of functional group
Code- di-(two) , tri-(three), tetra-(four)
List of prefixes
| No. of carbons | prefix | Alkyl Radical |
| 1 | Meth- | Methyl- |
| 2 | Eth- | Ethyl- |
| 3 | Prop- | Propyl- |
| 4 | But- | Butyl- |
| 5 | Pent- | Pentyl- |
| 6 | Hex- | Hexyl- |
| 7 | Hep- | Heptyl- |
| 8 | Oct- | ….. |
| 9 | Non- | …… |
| 10 | Dec- | …… |
Suffix, indicating the homologous series
| Homologous series | suffix |
| alkane | -ane |
| alkene | -ene |
| alcohol | -ol |
| Carboxylic acid | -oic |
Examples:
Example 1: An alkane made of 5 carbons:
Prefix→Pent- Suffix→ -ane so Name → Pentane
Example 2: An alkene made of 6 carbons:
Prefix→ Hex- Suffix→ -ene so Name→Hexene
- In the case of alcohol and carboxylic acid, you have to add an extra “an” after the prefix
- An alcohol made of 3 carbon atoms:
Prefix →Prop- (add an extra “an” after it) Suffix→- ol so Name→Propanol
Example 3: A carboxylic acid made of 2 carbon atoms:
Prefix→ Eth- (add an extra “an” after it) Suffix→ -oic so Name→ Ethanoic acid
Naming an Organic Compound by interprating the formula:
- C3H8 – the following formula satisfies the general formula of alkane, and consists of 3 carbon atoms. Name- Propane
- C5H10 – the following formula satisfies the general formula of alkene and consists of 5 carbon atoms. Name- pentene
- C3H7OH- the following is an alcohol as we can see a –OH group is attached to it, with three carbon atoms. Name- propanol
- C3H7COOH- the following is a carboxylic acid group as we can see a –COOH group attached, with 4 carbon atoms (we also include the carbon from the functional group when naming). Name- butanoic acid
Different types of formulas in organic chemistry
In organic chemistry, the organic compounds are usually expressed in three types of formulas:
- Molecular Formula
- Structural Formula
- Displayed Formula
To show the difference between them I am going to show butane in these three different types of formulas.
Molecular Formula
The molecular formula shows only the number of atoms present from each element. It provides no information about the type of bonding between the atoms. It also does not give any hint about the structure, like how the atoms are arranged in the molecule. Whether there is any sort of branching or not.
Example: C4H10
Structural Formula
Gives an idea about the structure, the compound is expressed as small groups of each carbon atom and hydrogen atoms attached to it, bonding is shown between each group by dashes, but the bonding between the carbon and hydrogen atoms is not shown.
Example: CH3-CH2-CH2-CH3
Displayed Formula
The displayed formula shows all the individual bonds in an organic compound. You will even have to show the bonds between the individual carbon and hydrogen atoms.
If there is alcohol, you will have to show the bonding between the hydrogen and oxygen atom.
Usually, in IGCSE chemistry exams, there are two mark questions that ask you to draw the displayed formula of specific organic compounds.
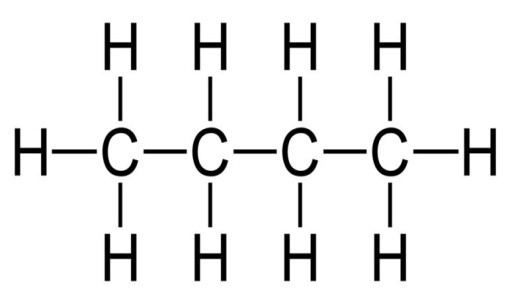
Structural Isomerism
Up to now, all the organic compounds we have seen, all the carbon atoms were neatly lined up in a straight line. But in reality that might not be the case.
In reality, instead of following one carbon after another, they can form branches. D
It is not considered a valid branching if the alkyl group is connected to the end carbon atoms.
We count the position number from the end where it would be less.
I know this is a bit confusing. Please have a look at the examples below, I strongly believe they will clear things up! Also please refer to the Alkyl radical table above.
Isomers
Isomers are simply organic compounds with the same molecular formula but different structural formulas.
To simply put isomers are different structural variations of the same organic compound.
For example, the molecular formula of
Examples:
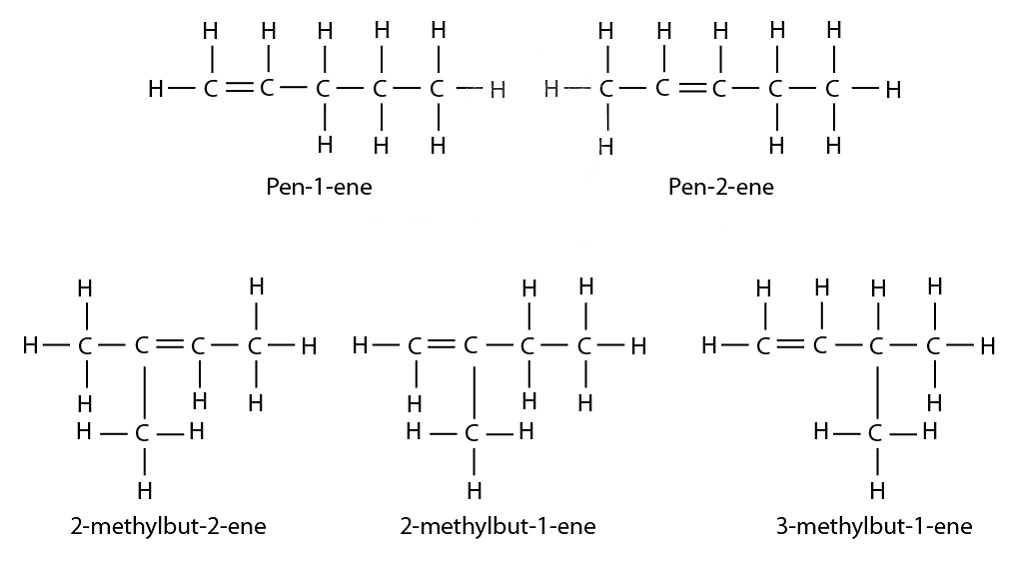
Isomers of Pentene
- I have listed below all the different Isomers of pentene (C5H10) along with names and displayed formula.
- Please remember, when you are naming an isomer. Make sure to mention the position number of the carbon to carbon double bond. Also, mention the position number of the alkyl radical.
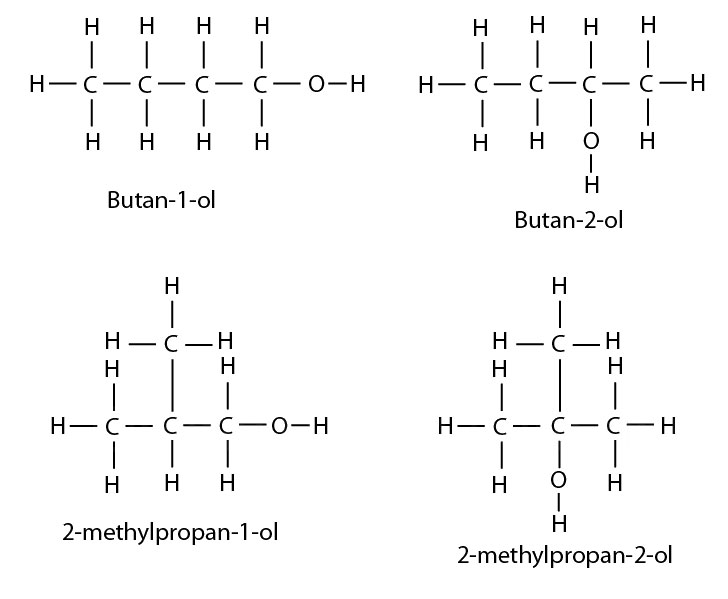
Isomers of butanol:

Physical properties of Alkanes, Alkenes, alcohols and carboxylic acids:
Organic compounds which are in the same homologous series show a similar trend in physical properties.
For example, the melting and boiling points of alkanes increases as their carbon number increase (or their size increases). Because as their size increases the intermolecular forces between the molecules increases. Hence takes more energy to overcome. Therefore the lighter alkanes are usually gas at room temperature while the heavier alkanes are liquid. To learn more about intermolecular forces, please view this article.
The alkenes also show the same physical trend due to the same reason. But even though the trend is similar all the alkenes comparatively have lower melting and boiling points than alkanes. This is because due to the carbon to carbon double bonds, the intermolecular forces are not that strong as it is in alkanes.
The alcohol and carboxylic acids have much higher melting and boiling points than both the alkanes and alkenes. This is due to their ability to form strong hydrogen bonding between the molecules as they are the -OH
All the organic compounds which are in the same homologous series have similar chemical properties.
Chemical Reactions of Alkane, Alkene, and Alcohols:
Alkanes: alkanes are the most inert of all the homologous series, due to the presence of only C-H bond which is quite stable or inert.
The alkanes undergo only two types of reactions combustion with oxygen/air and substitution reaction with halogens.
| Reaction name | Reaction(with an example) and conditions(if any) |
| Combustion (reaction with oxygen) | Alkane+ Oxygen→ carbon dioxide+water
CH4(g) + 2O2(g)→ CO2(g) +2H2O(l) (insufficient supply of oxygen causes incomplete combustion and produces carbon monoxide or soot(carbon) instead of carbon dioxide |
| Substitution (reaction with halogens) | Alkane + Halogen→ Hydrogen Halide+ Halogenoalkane
CH4(g)+Br2(g)→CH3Br(g)+HBr(g) Condition: the presence of UV light |
Important
Alkenes: alkenes are more reactive than alkanes due to the presence of carbon to carbon double bond as the functional group. If there is any carbon to carbon double present in an organic compound, it is said to be unsaturated. If no carbon to carbon double bond is present in an organic compound, then it is called saturated.
Here are the two basic reactions of alkenes; another one is during the production of alcohol.
| Reaction name | Reaction(with example) and conditions (if any) |
| Combustions (reaction with oxygen) | Alkene+oxygen→Carbon dioxide+water
C2H4(g)+O2(g)→ 2CO2(g)+2H2O(l) |
| Addition(reaction with halogens) | Alkene+Halogen→dihalogenoalkane
C2H4(g)+Br2(l)→ C2H4Br2(l) |
Test of Unsaturation (presence of carbon-carbon double bond)
Test for unsaturation means testing whether carbon to carbon double bond is present in an organic compound. This is an extremely common question that frequently appears in IGCSE chemistry exams.
Bromine is water is simply water with bromine mixed with it. When bromine mixes with water it forms an orange-brown solution, which is called bromine water.
When an organic compound is unsaturated (contains carbon to carbon double bond). If you add bromine water to it, then the bromine water will be decolorized. In simple words, it would turn from orange-brown to colorless.
Alcohols: Two basic reactions of alcohols are combustion(reaction with oxygen) and dehydration(loss of water from the compound) :
| Reaction name | Relevant equations and conditions |
| Combustion | Alcohol+oxygen→ carbon dioxide+ water
C2H5OH(l)+3O2(g)→2CO2(g)+3H2O(l) |
| Dehydration | Alcohol→Alkene+water
Conditions: heat, aluminum oxide as catalyst Ethanol→Ethene+ water C2H5OH(g)→ C2H4(g)+ H2O(l)
|
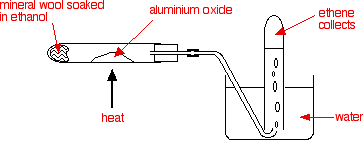
Production of ethanol
There are two reactions by which ethanol is mass-produced, Hydration and Fermentation. Here are the conditions and reactions that occur in these two processes.
For an in-depth explanation about the production of ethanol by fermentation, you can also have a look at my blog “Production of Ethanol from Sugarcane“
| Process name | Relevant equations and conditions |
| Fermentation | Yeast is mixed with starch/sugar solution, and left in a sealed container, at 30-40◦C, the yeast respires anreobically, produces an enzyme that converts glucose to ethanol and carbon dioxide gas
C6H12O6→ 2C2H12OH(aq) + 2CO2(g) |
| Hydration | Ethene+ steam→ethanol
Temperature:300◦C, Pressure: 60-70 atm Catalyst: phosphoric acid C2H4(g) + H2O(g) ➝ C2H5OH(g) Only a small portion of ethene reacts. The ethanol produced is condensed as a liquid and the unreacted ethene is recycled. |
Comparison between fermentation and hydration of ethene
| Fermentation | Hydration of ethene | |
| Use of resources | Uses renewable sources- sugar beet or sugar cane, corm, or other starchy materials. | Uses finite resources- once all oil has been used up there won’t be any more |
| Type of process | A batch process- everything is mixed together in a reaction vessel and then left for several days. That batch is then removed and a new reaction is set up-this is inefficient | A continuous process- a stream of reactants is constantly passed over the catalyst. This is more efficient than a batch process. |
| Rate of reaction | Slow, taking several days for each batch | rapid |
| Quality of product | Produces very impure ethanol that needs further processing | Produces much purer ethanol |
| Reaction Conditions | Uses gentle temperature and ordinary pressures | Uses high temperatures and pressures, needing a high input of energy. |
Ethanol is used as biofuel, it is renewable and produced by fermentation. But it is not such a bright idea to plant crops for fuel instead of food.
What is crude oil (petroleum)?
Crude oil, also known as petroleum is a viscous
For a better and more in-depth explanation of how different fractions in crude oil are separated, please read my blog “How gas and diesel is separated from crude oil“
By hydrocarbon, I simply mean alkanes and alkenes. Crude oil simply contains alkanes and alkenes of various sizes, all mixed together.
Properties of hydrocarbons as their size increases
As the size of the hydrocarbon increases, the intermolecular forces between the molecules increase. This is because, as the size of the molecules increases, the intermolecular forces between them also increase (to learn more about intermolecular forces, please view this blog post). This results in the following:
- High melting and boiling point
- The liquids becomes less volatile (evaporates less easily)
- The liquid becomes viscous
- Bigger hydrocarbons do not burn so easily as the smaller ones. This limits the use of bigger ones as fuels.
Separation of crude oil
The different fractions of crude oil are separated by the process of fractional distillation. The crude oil is heated and inserted into a fractionating column. Fractions of the crude oil that have lower boiling points, turn to gas and move up the fractionating column. The fraction of the crude oil having a higher boiling point remains at the bottom which the ones with lower boiling points turn to gas and move up.
This is how different fractions of the crude oil are collected at different heights of the fractionating column.
Please view the diagram and the table below to learn more about the different fractions of crude oil and how we use them.
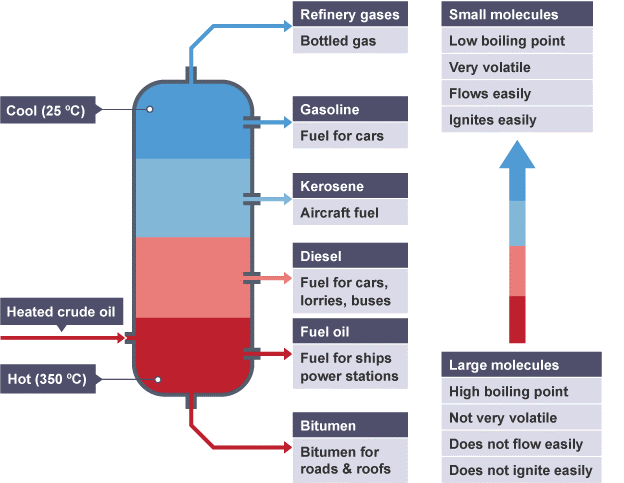
| Fraction | Use |
| Refinery gas | A mixture of methane, ethane,propane, butane, in LPG(Liquefied Petroleum Gas), used for domestic heating and cooking |
| Gasoline | Used as fuels for cars |
| kerosine | Used as aviation fuel, domestic heating |
| Diesel(Gas oil) | Used for buses, trucks, lorries, trains |
| Fuel oil | Used for ships and industrial heating |
| Bitumen | To make roads |
Cracking
cracking is a useful process in which large hydrocarbon molecules are broken into smaller ones.
The big hydrocarbon molecules in gas oil, for example, can be broken down into smaller ones needed for petrol.
For an in-depth and simplified note about cracking please have a look at my blog “Why major oil and gas companies use cracking to produce gasoline“.
Why cracking?
There is a very high demand for lighter hydrocarbons such as petrol because they burn easily. But in crude oil, there are not enough fractions of the lighter hydrocarbons to provide for this demand. To fulfill this high demand, the larger fractions of crude oil are cracked to produce extra lighter hydrocarbon,s. The larger fractions are quite unreactive and cannot be used as fuel, for this reason, they must be cracked.
Cracking also produces hydrocarbons with carbon to carbon double bonds (alkene), which are very useful as they are quite reactive. These are used by the chemical industries to produce different types of chemicals such as shampoos, pesticides, etc.
How cracking works?
The larger fraction of hydrocarbon is converted to gas by heating, then passed over the catalyst of mixed silicon dioxide and
Cracking is a random process, so the smaller fractions produced during cracking might not be the same.
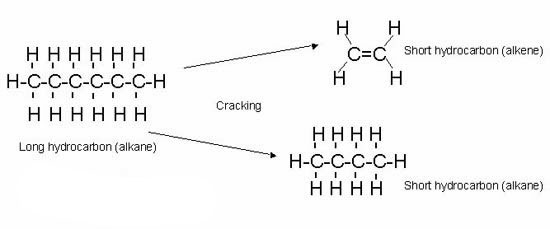
Cracking produces a mixture of alkanes and alkenes.
For example, a large alkane C13H28 can breakdown into several combinations of alkanes and alkenes
C13H28 → C2H4 + C3H6 + C8H18
C13H28 → 2C2H4 + C9H20
C13H28 → 2C2H4 + C3H6 + C6H14
C13H28 → 2C2H4 + C3H6 + C6H12 + H2

Polymers
Polymerization is the process of joining small molecules called monomers to form a big molecule called a polymer.
There are two types of polymerization, addition polymerization, and condensation polymerization.
Addition Polymerisation
In addition polymerization, the monomers are unsaturated (have carbon to carbon double bonds). The monomers are of the same types; they join up to form a polymer. There is no byproduct. For a more advanced blog post on addition polymer, please view this blog.
Condensation Polymerisation
In condensation polymerization, the polymers are of two types, in this type of polymerization, water is produced as a byproduct.
The naming of addition polymer, Poly(monomer name)
Examples of addition polymers
Poly(ethane):
Monomer: ethene (C2H4)
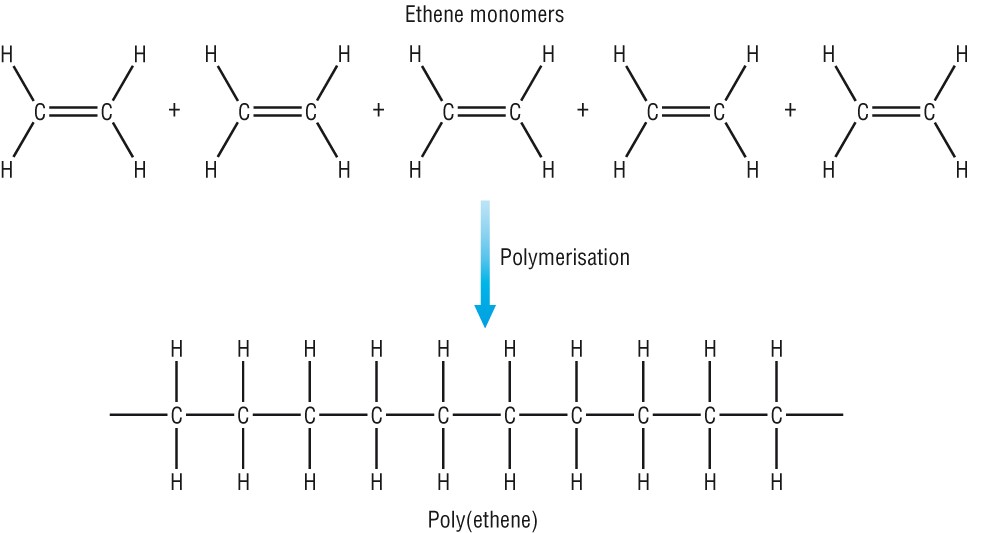
In terms of repeating unit
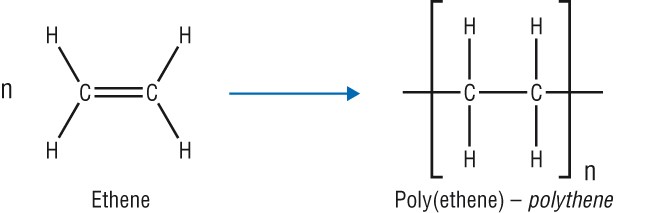
There are two types of poly (ethene):
Low density poly (ethene) [LDPE] : soft, flexible
High density poly(ethene)[HDPE]: more rigid
Uses of LDPE: shopping bags
Uses of HDPE: plastic bottles
Poly(propene):
Monomer: propene
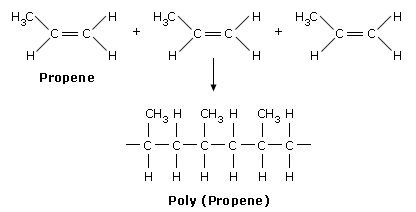
In terms of repeating units:
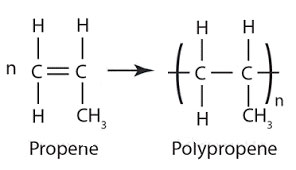
Uses of poly(propene): to make crates, ropes [stronger than poly(ethane)]
Short form: PP
Poly(chloroethene)/PVC(poly vinyl chloride)
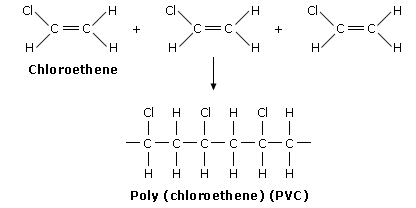
In terms of repeating unit:
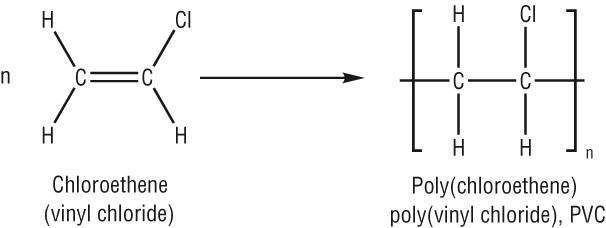
PVC is quite rigid
Uses of PVC: drain pipes, replacement windows, floor covering sheets, an electrical insulator.
Examples of condensation polymers:
Nylon:
Monomers: hexanedioic acid (HOOCCH2CH2CH2CH2COOH)
Two carboxylic acids (-COOH) groups attached at both ends
1, 6-diaminohexane (H2NCH2CH2CH2CH2CH2CH2NH2)
Two amines (-NH2) groups attached at both ends
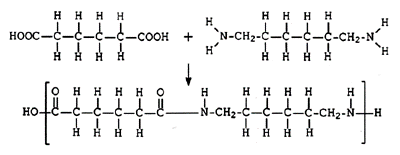
Uses of nylon: textiles, ropes, cogs, and bearings for machines
Conclusion
So this is my version of an in-depth note on IGCSE organic chemistry. I tried to cover everything related to organic chemistry but also made sure to keep it within the boundaries of IGCSE chemistry.
If you have found this blog post helpful, then please feel free to share this blog post with your friends who desperately need help with organic chemistry.
Some organic chemicals can be Carcinogenic and might cause cancer. For those cases, it is better to learn about some Life and health insurance packages. Click here to learn more.
Looking for IAL Alcohols, click here

These notes are very helpful and saved me during my exam. Thank you for these fabulous notes on organic chemistry
These organic chemistry notes were very helpful and it saved a lot of my time. I think under the subheading “Production of ethanol”, in the row labelled “Hydration”, it should be ethene and not ethane. Even in the chemical reaction of the same concept, the formula of ethanol formed seems to be incorrect and is unbalanced.
Hi Manan Siroya, thank you so much for pointing out the mistake. I have corrected it, it really helps others out who are using this blog post. Thank you so much!
Can I get a A* in my organic chemistry exam if I remember everything in this blog?
If you are taking your IGCSE chemistry exam then it would help you a lot.
Do we need to know about different linkages in this chapter for the IGCSE chemistry exam?
Is this possible to make a downloadable copy of these notes. I’d like to print them out to read.
Yes, I am working on this to implement it soon.
A very comprehensive note on IGCSE Organic Chemistry. It is very helpful for the students. I am recommending my student to go through these notes for a better understanding.