Intermolecular forces are weak attractive forces between molecules. There are three types of intermolecular forces. The first one is called London forces, also known as dispersion forces the second one is dipole-dipole interaction and finally, the third one is Hydrogen bonding. London forces are the weakest whereas the Hydrogen bonding is the strongest intermolecular force.
London Forces:
This is the type of intermolecular forces which exists between nonpolar molecules. It is the weakest intermolecular force.
Other names of London forces: Van der Waals forces, instantaneous-instantaneous dipole forces, dispersion forces.
How do London forces work?
Suppose we have a non-polar molecule. Randomly at an instant, the electrons in the atom may not be evenly distributed around the atom. Most of the electrons may go to one side, making that side partially negative, on the other side there is lack of negative charge, the nucleus is exposed more., there it becomes partially positive. This type of diploe produced is called instantaneous dipole. It is a very random process.
The molecules next to ones with instantaneous dipole will have an induced dipole moment. Then there will be an attraction between the molecules. Sometimes these may become zero.
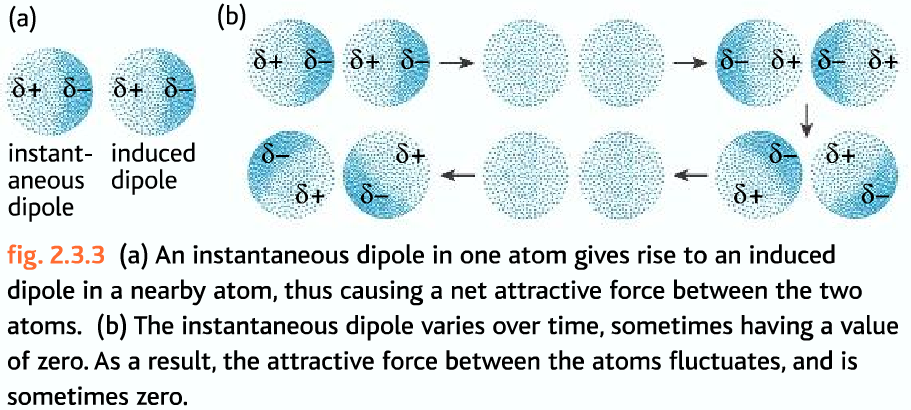
The size of this forces increases as the number of electrons in the molecule increases. More electrons mean more of this effect. More mass means more electrons, so as the mass of molecules increases the size of the London forces increases.
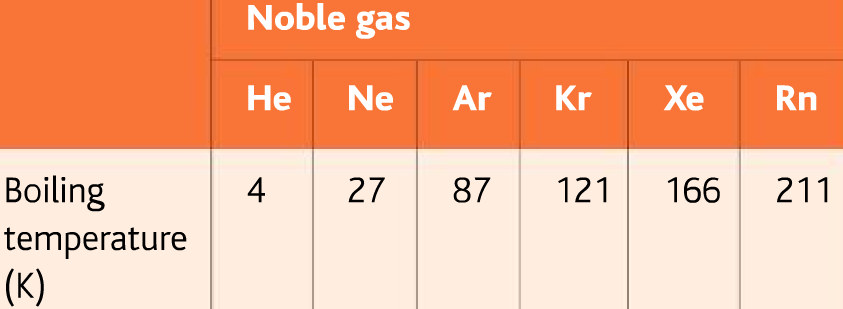
Examples of substances with only London forces present: chlorine, fluorine, hydrogen, alkane, alkenes etc.
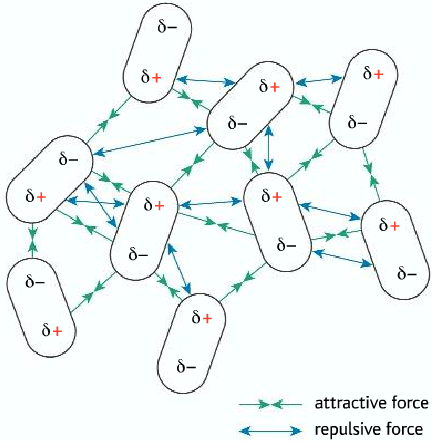
Dipole-Dipole Interactions
- This type of interactions occurs between molecules with net/permanent dipoles (polar molecules)
- The negative side of the molecule is attracted towards the positive side of the other molecule
- It is stronger than London forces
- Substances with polar molecules have both dipole-dipole interactions and London forces.
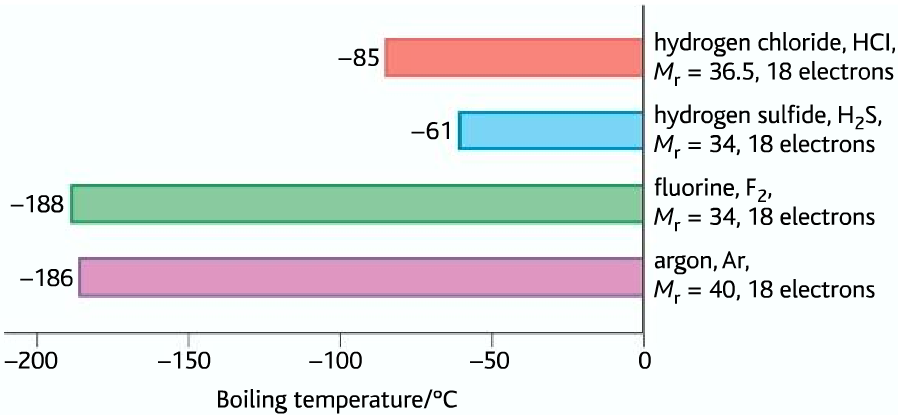
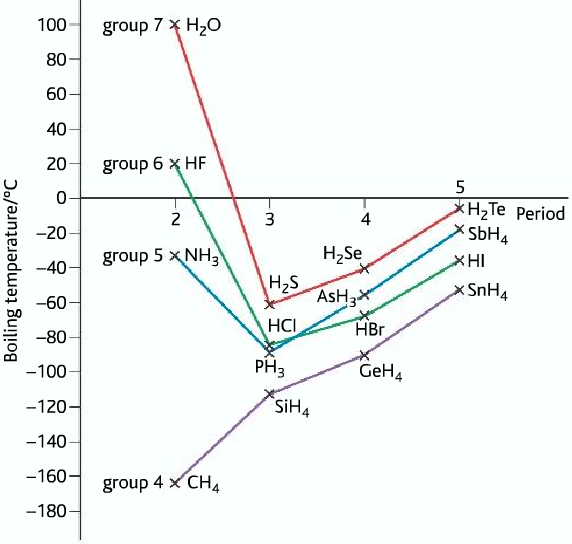
Boiling temperature comparison
Hydrogen Bonding
- Hydrogen bonding is the strongest type of intermolecular force.
- Fluorine, oxygen, and nitrogen are top three most electronegative element.
- Also is the least electronegative element.
- So when these three elements form covalent bonding with hydrogen (0-H,H-F,H-N), the resulting bond is highly polar. Hydrogen which has only one electron, the electron density is drawn from it, leaving the positive nucleus of hydrogen exposed.
- So when there is a lone pair in another molecule. There can be a strong attraction between the lone pair and the exposed positive part of hydrogen. Such high attraction is called hydrogen bonding.
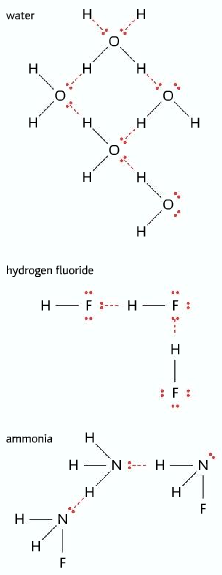
- Alert: Hydrogen bonding can only be formed if O-H, H-F or N-H bonds are present.
- Common substances where hydrogen bonding is formed: H2O , HF, NH3 , alcohol, carboxylic acid
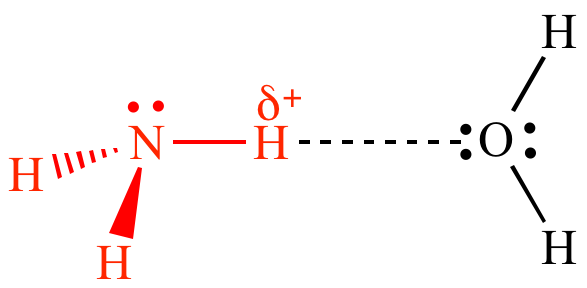
Explaining some substances with a high boiling point
Substances like water, hydrogen fluoride and ammonia have higher boiling points than compounds with more mass, for which they are liquid at room temperature while the others are gases. This is because there is strong hydrogen bonding in these compounds which takes a very high energy to overcome.


Great tremendous things here. I’m very glad to look your article. Thank you so much and i am taking a look ahead to contact you. Will you please drop me a mail?