The polymer is a long chain molecule which is formed by joining many small molecules called monomers. Addition polymers are a type of polymer in which all the monomers are unsaturated or to simply to say are alkenes. During the polymerization process, the double bonds in the alkenes break and they all join together to form a large molecule.
Types of addition polymers
Poly(ethene)
Monomer: ethene
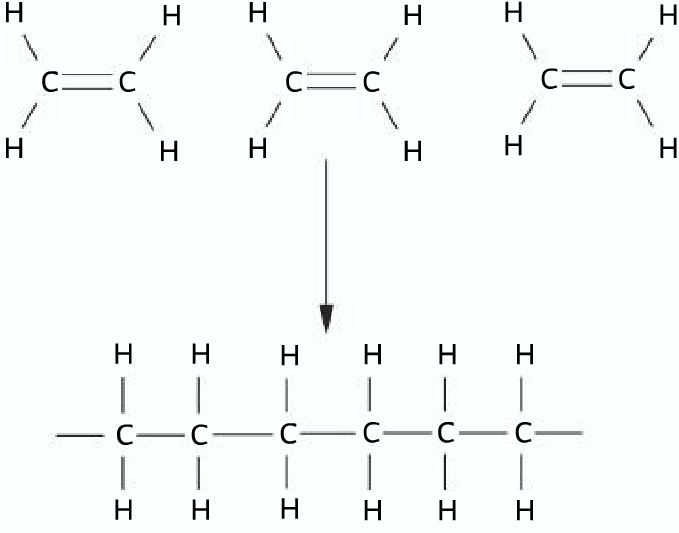
Repeating unit:
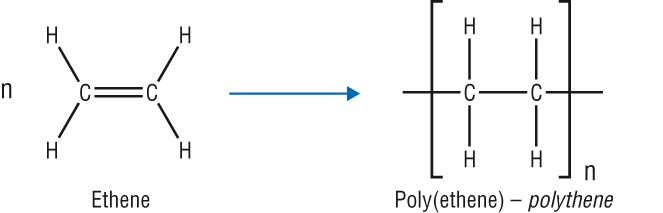
- There are two types of poly(ethene):
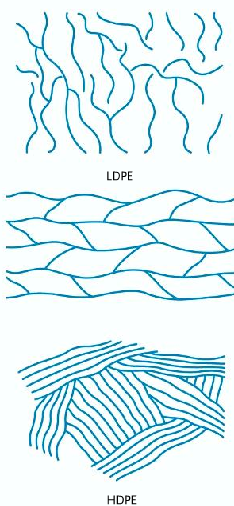
LDPE (Low-Density Polyethene) and HDPE (High-Density Polyethene)
LDPE:
- This kind of poly(ethene) is soft, malleable with a low melting point due to its high branching.
- It is made in the condition of 200°C and 1200 atm pressure
- It is mainly used for packaging and forming utensils
HDPE:
- This kind of poly(ethene) is more rigid with a higher melting point than LDPE. It is also denser than LDPE.
- This kind of poly(ethene) is made by using low temperature of 60°C, catalysts and 1 atm pressure.
Both types of poly(ethene) softens when heated.
Ploy(chloroethene)/PVC (Poly Vinyl Chloride):
Monomer: Chloroethene
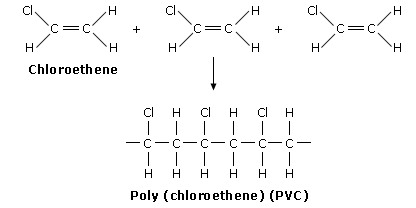
Repeat Unit:
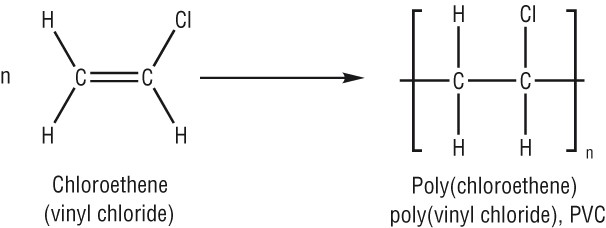
- This kind of polymer is more rigid due to the presence of a polar C-Cl bond, causing greater intermolecular forces.
- It is used to make ropes, sanitary pipes etc
Plasticizer makes it softer, so it can be easily worked with.
What factors affect properties of polymers?
Properties of polymers: softness, flexibility, melting point, tensile strength etc.
- The average length of polymer chain: As the chain length of a polymer increases the melting point and tensile strength increases.
- Branching of chains: The greater the branching in a polymer chain the less the melting point and tensile strength of that polymer and also lower density
- Intermolecular forces between chains: the higher the intermolecular forces between chains, the higher the melting point.
- Cross-links between chains: the stronger the bonding between two chains the higher the melting point, rigidity and hardness.
Problems with polymers
- Polymers have high energy production costs
- Polymers uses up non-renewable resources (e.g: ethane is obtained from crude oil)
- Disposal problems, because polymers are non-biodegradable, so it uses up lands. Also when polymers are burned toxic gases are released.
Non- biodegradable: cannot be broken down by the action of bacteria or other micro-organisms.
What is carbon footprint?
Ans: It is the amount of carbon dioxide released into the atmosphere from the activities of an individual, organization or community.
- It is measured as units of carbon dioxide
- Suppose you buy a plastic bottle, when you throw it away it would be burned and carbon dioxide would be released. The amount of carbon dioxide released would add to your carbon footprint.
Solutions to polymer problems
- Using renewable energy sources: the energy required to make polymers is obtained from renewable energy supplies such as wind power, solar etc. This reduces the environmental effects.
- Reducing use of polymer products: using less polymer products would decrease the carbon footprint. Suppose using paper bags instead of polymer bags, using paper cups and plates instead of using polymer ones.
- Recycling: Reusing polymer products such as plastic bottles or bags would reduce burning of those products and reduce carbon footprint. Also making new products out of one polymer product is another form of recycling. For example recycling plastic bottles to make carpet or car body parts.
Ways of Recycling plastic
- Mechanical Recycling: In this process, the plastic is shredded and turned to granules before using it to make new items. But here the problem is that the plastic needs to be separated by hand which can be expensive.
- Chemical Recycling: In this process, the plastic is broken down to monomers, which are again joined to make new polymer. But this is very expensive as it requires a high energy input.
Biodegradable polymers
- Making biopolymers, bioplastics or green plastics
- This kind of polymers can be made from renewable sources such as starch and cellulose, which are obtained from crops such as sugarcane or maize
- It reduces carbon footprint because when the crops are regrown it absorbs carbon dioxide
- This kind of polymers can be broken down by the action of microorganisms such as bacteria.
Energy Recovery
In the simplest form of energy recovery, the polymer is burned to produce heat energy. The heat energy is used to turn water to steam to turn turbines and produce electricity. Also, this heat can be used to heat water.
Another option is turning polymers into fuels, there are two ways it can be done: pyrolysis and gasification.
- Pyrolysis: in this process, the polymer is heated in the absence of oxygen, it produces fuel which can be burned to get energy.
- Gasification: In this process, the polymer is heated in limited oxygen. It produces syngas (a mixture of hydrogen and carbon monoxide), it is the raw material for various important reactions.
Life Cycle Analysis
- It is done to find out any harmful effect the polymer might have to the environment.
- To identify any improvements which can be made.
To learn more about Polymers and their various uses, you can check out this book “Basics of Polymer Chemistry (River Publishers Series in Polymer Science)” from Amazon.


plez can help tell me the method preparation of (MMA) & (PMMA)& Silicone rubbers and flexible risen
Thanks a lot for the great content. I have shared it on my facebook page.