Alcohol definition
Alcohol is one of the most important homologous series in organic chemistry, it is simply a homologous series, an alkyl group R, with a –OH group attached to it as the functional group. This post is going to cover alcohol names, their formula, reactions, and types of alcohols.
Alcohol can be represented by R-OH
Where R can be a methyl (-CH3) , ethyl (-C2H5) , propyl (-C3H7) etc.
Name of an alcohol ends with an –ol (suffix)
Example: butanol
At this point in the blog if you have no idea what I am talking about, this means your concepts with organic chemistry are very weak. In that case, you should have a look at this book “Organic Chemistry I For Dummies (For Dummies (Lifestyle))” from Amazon.
Types of alcohols
Alcohols can be divided into three types, depending on their structure, primary, secondary and tertiary.
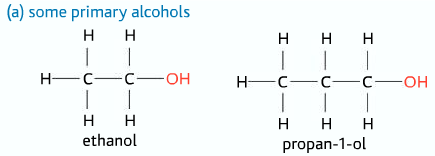
Primary Alcohols
Only one alkyl group and two hydrogen atoms attached to the carbon atom to which the –OH group is attached to
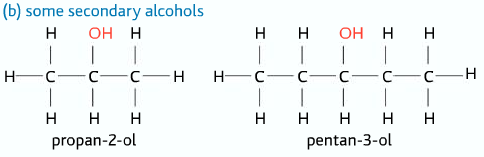
Secondary Alcohols
Two alkyl groups and one hydrogen atom attached to the carbon atom to which the –OH group is connected.
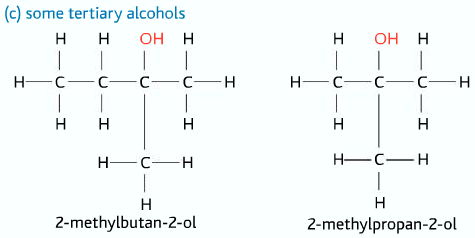
Tertiary Alcohols
Three alkyl groups and no hydrogen atom attached to the carbon atom to which the –OH group is connected to.
Reaction of alcohols
Combustion of alcohol:
Alcohol + Oxygen –> Carbon dioxide + water
The reaction of sodium with alcohol
Reaction of sodium with water |
Reaction of sodium with ethanol |
|
|
Reaction of alcohol with phosphorous pentachloride (PCl5)
CH3CH2OH (l) + PCl5(s) –> CH3CH2Cl(l) + POCl3(l) + HCl(g)
The HCl(g) formed during the reaction, forms white forms white fumes with the water vapor in the air.
Test for alcohols
add solid PCl5
Result
White fumes would be formed
Oxidation of primary, secondary and tertiary alcohols
- The alcohols are oxidized using the oxidizing agent potassium dichromate (K2Cr2O7) as a typical oxidizing agent, it itself is reduced from orange dichromate (VI) to dark green chromium(III) ions.
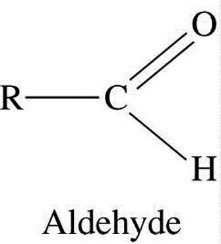
- The aldehyde is a homologous series with –COH group attached.
- The ketone is a homologous series with C=O group between two alkyl groups
Oxidation of primary alcohols
- Primary alcohol firstly undergoes oxidation to produce aldehyde.
- On further oxidation of the aldehyde, carboxylic acid is produced
- Primary alcohol à Aldehyde à Carboxylic acid
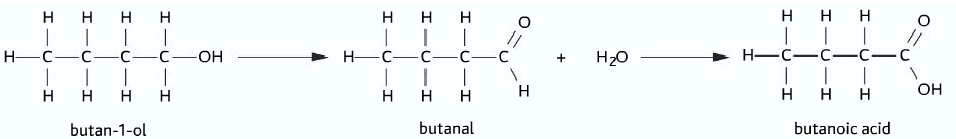
Oxidation of secondary alcohols
Secondary alcohol oxidizes only once to produce the corresponding ketone.
Secondary alcohol –> ketone
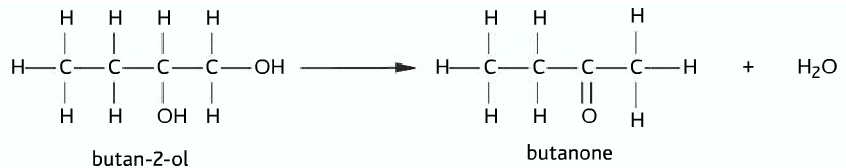
Tertiary Alcohols
Do not undergo oxidation at all
Differentiating between the primary, secondary and tertiary alcohols
The primary and secondary alcohols turn orange potassium dichromate to green, while the tertiary alcohol does not have any effect on the potassium dichromate, it remains orange.
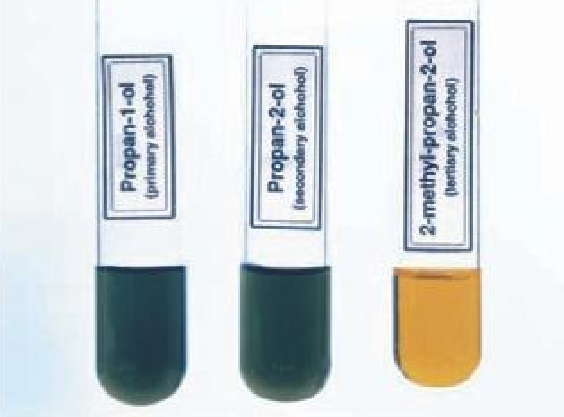
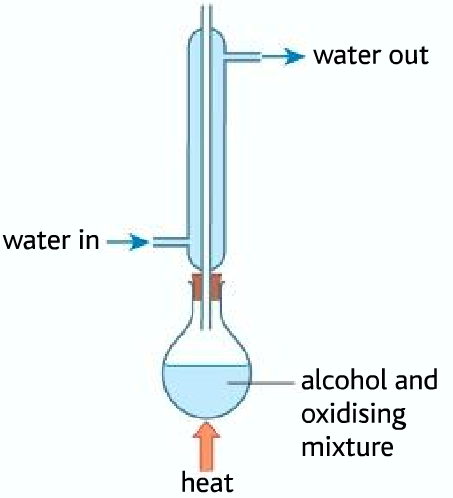
Obtaining aldehyde from the oxidation of primary alcohols
Below are given the boiling points of alcohol, aldehyde, and carboxylic acid
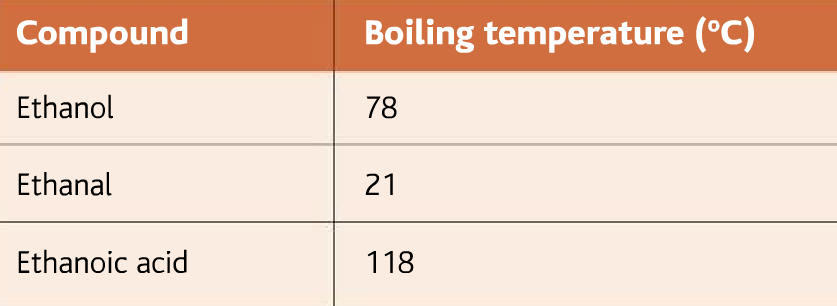
As it can be seen from the table, that the aldehyde has the least boiling point. As soon as the aldehyde is produced it boils. The aldehyde, therefore, can be collected by the fractional distillation method.
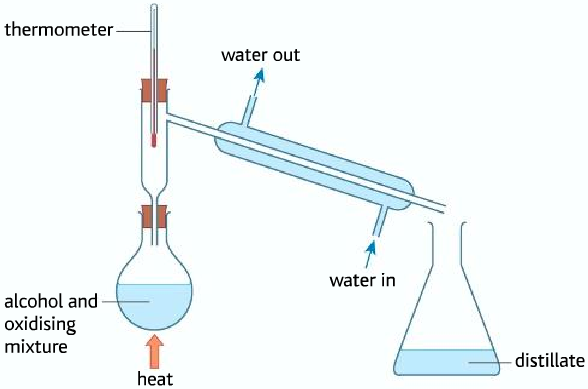
Obtaining the carboxylic acid from the oxidation of primary alcohol
- In order to obtain carboxylic acid, the aldehyde produced must be oxidized further by the potassium dichromate. But the problem is that the aldehyde vaporizes due to the heating before it can be oxidized to form the carboxylic acid.
- To make sure that the aldehyde remains in the flask to be oxidized to the carboxylic acid, a reflux condenser is used. The reflux condenser condenses the aldehyde back into the flask so that it is oxidized to the carboxylic acid.
- Obtaining carboxylic acid from primary alcohols
At this point, after reading this blog, if you need to know even more about alcohols and their reactions, then I would recommend you read page number 542 of this book: Organic Chemistry (9th Edition) (MasteringChemistry)


These notes are awesome just shared it with all my classmates who are struggling to pass chemistry ❤️❤️
Thank you very much! I am working to upload more notes.
Please upload more Ial chemistry notes really great full to you