What is Rate of Reaction?
The rate of reaction is simply the speed at which a reaction takes place. There are several factors that affect the rate of reaction such as surface area, temperature, concentration, and the catalyst. This post will show how these factors affect the rate of reaction using the reaction between calcium carbonate and hydrochloric acid.
The reaction between Calcium Carbonate and Hydrochloric acid
When calcium chloride reacts with hydrochloric acid, calcium chloride, water, and carbon dioxide are produced. The carbon dioxide produced leaves the mixture as a gaseous product.
CaCO3(s) + 2HCl(aq) → CaCl2(aq) + H2O(l) + CO2(g)
The rate of reaction is nothing but the rate at which products are produced or the reactants are used up. If the rate of production of a certain product with respect to time, we get an idea about the rate of reaction. Here we are going to track the rate at which the carbon dioxide gas is being produced.
Goal: We are simply going to find out the rate at which the carbon dioxide gas is being produced with respect to time. Remember, as the carbon dioxide gas produced escapes the reaction mixture, there is an overall decrease in mass.
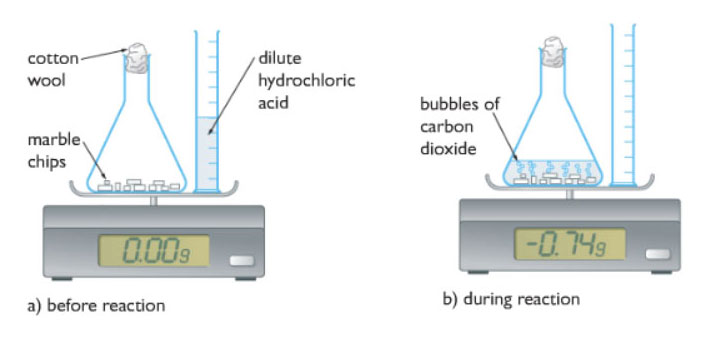
The top pan balance as shown in the diagram measures the change in mass. As we can see in the diagram, everything is placed on the pan and the mass is set to zero. Then we pour the dilute hydrochloric acid into the flask which contains the marble chips or calcium carbonate to be exact, the reaction then starts immediately
As the reaction proceeds, carbon dioxide gas is produced. The cotton wool is placed at the mouth of the conical flask to avoid spraying out of solution and an unnecessary decrease in mass. As the carbon dioxide gas escapes the reaction mixture the overall mass decreases as shown by the negative mass.
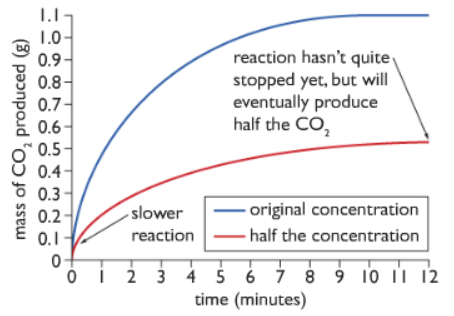
The negative mass directly indicates the mass of carbon dioxide which has escaped. As the reaction proceeds the negative mass increases, showing that more carbon dioxide is produced. Finally, the negative mass stops increasing as the reaction ends indicating that no more carbon dioxide gas is being produced.
To learn more about the rate of reaction, you can check out this awesome book “Chemistry: Reaction Rates and Equilibrium (Home Use)” on Amazon.
The mass of carbon dioxide is plotted against time in a graph as shown below:
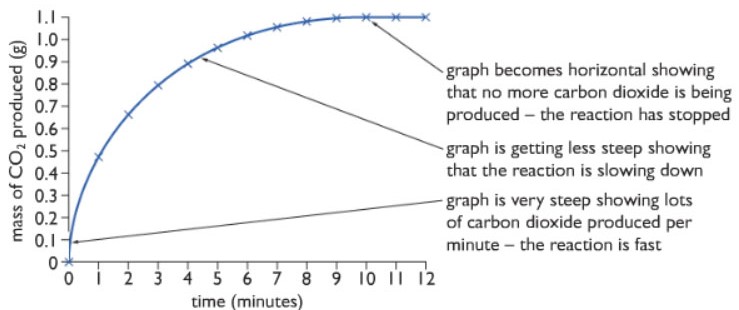
Observation: The steepness of the gradient of the graph measures the rate of reaction. As we can see from the diagram, at the beginning of the reaction, the gradient is the highest, meaning the rate of reaction is highest at the beginning. But as the reaction proceeds the steepness decreases meaning the rate of reaction decreases till we get a flat horizontal line. This indicates that the rate of reaction is zero and the reaction is over.
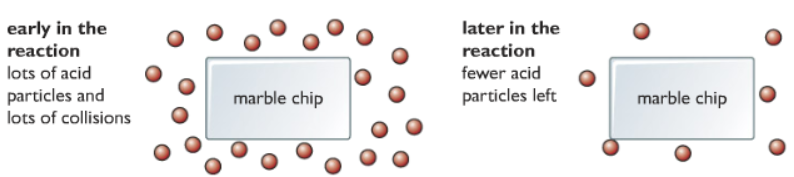
Why the rate of reaction is highest at the beginning and falls as the reaction proceeds?
Answer: Explaining in terms of collision theory, reactant particles need to collide for the reaction to happen. At the beginning of the reaction, there are plenty of reactant particles, they collide with each other more often hence the rate of reaction is high. As the reaction proceeds the reactant particles get used up, they collide with each other less often, and hence the rate of reaction decreases.
Using different reaction conditions to see how it affects the rate of reaction
1) Using smaller marble chips:
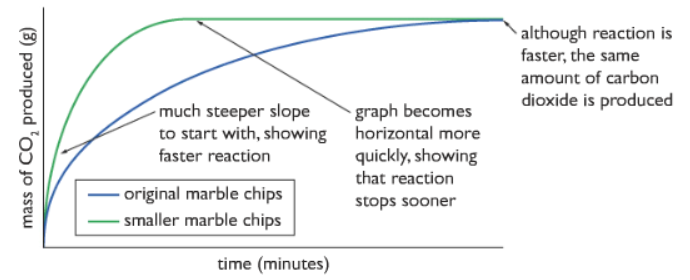
As we can see from the graph, the curve for the smaller marble chips is much steeper than the one with the bigger marble chips, which means that the rate of reaction is higher for the smaller marble chips used. Because using smaller marble chips increases the surface area, meaning that more of the solid is exposed to the acid particles. The amount of carbon dioxide produced is the same because the acid is limited.
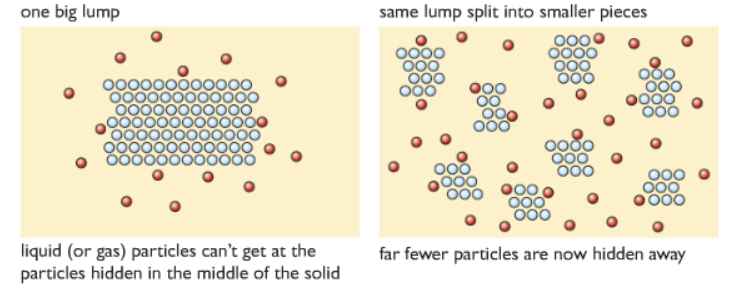
2) Changing the concentration of the acid:
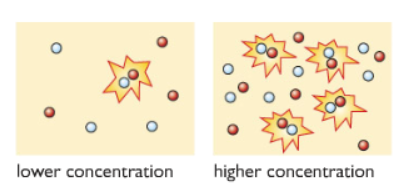
Explaining in terms of collision theory:
i) Higher Concentration: The rate of reaction is higher because there are more number of acid particles, they collide with each other more often with the marble chip particles. The product produced is also more because there are more acid particles present.
ii) Lower Concentration: The rate of reaction is lower for a lower concentration of acid because less number of collision occurs hence the rate of reaction decreases.
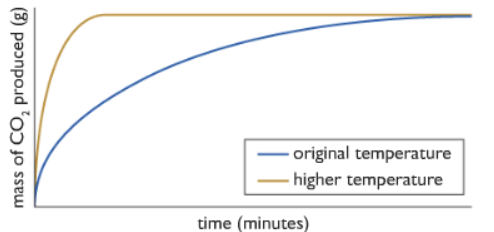
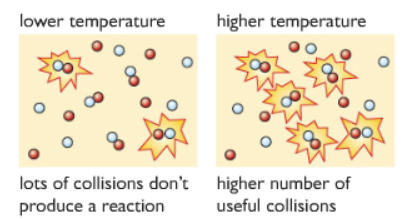
3) Changing the temperature:
Useful Collisions: For reactant particles to collide and react, the particles must have minimum energy called the activation energy. If reactant particles have energy below that, they just collide and bounce off each other. If the particles have energy more or equal to the activation energy then the reaction occurs.
At Low temperature: most particles collide with each other, but as most of them do not have enough energy equal to or more than the activation energy, they simply bounce off of each other.
At Higher temperature: the particles move faster, collide with each other more often, collision frequency increases. The particles also have energy equal to or more than the activation energy. So more number of useful collision occurs.
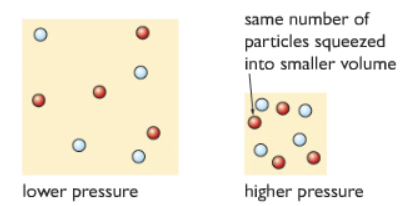
4) Changing the pressure:
Changing the pressure only affects the reactions with at least one gaseous reactant. Increasing the pressure causes the reactant particles to be closer. They collide with each other more often and hence increase the rate of reaction.
5) Using a catalyst:
Catalysts are chemicals that increase the rate of a reaction without being used up itself. So catalysts simply increase the rate of reaction.
Showing that a substance is a catalyst:
- A catalyst visibly increases the rate of reaction
- At the end of the reaction, it is neither used up or changed
Example: showing that Manganese(IV)Oxide is a catalyst, in the decomposition of hydrogen peroxide.
When the manganese (IV) oxide is added to the hydrogen peroxide solution, we can visibly see more bubbling of oxygen gas, visibly showing that it has increased the rate of reaction.
Before the reaction, the manganese(IV)oxide was weighed, after the reaction, the manganese(IV) oxide was dried and reweighed, the mass was found to be the same, hence showing that it remained unchanged after the reaction.
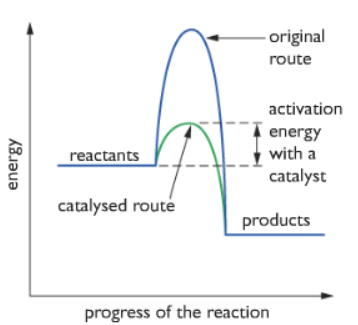
How does a catalyst work?
Answer: The catalyst provides an alternative route for the reaction to occur which has less activation energy. So more particles have energy equal to or more than the activation energy so more number of effective collision occurs.

Pretty section of content. I just stumbled upon your weblog and in accession capital to assert that I acquire actually enjoyed account your blog posts. Anyway I’ll be subscribing to your feeds and even I achievement you access consistently quickly.
Thank you so much that you have found my contents helpful! Please bookmark this website for now.
I really can’t believe how great this site is. Keep up the good work. I’m going to tell all my friends about this place.
Thanks, Perry, please share this website on social medias.
This site looks better and better every time I visit it. What have you done with this place to make it so amazing?!
Thank you! I am always working to make the site more user-friendly.
I’m working my way through your post. Chemical Engineering is my major, but I’m retired and simply seek to keep the challenge in front of me. Thank you for your outstanding effort.
Thanks, Wayne. I will update the blogs with more information.