What is crude oil and how it is formed?
Crude oil, also known as petroleum is a viscous brown liquid which is extracted from underground or under the seabed. It is a mixture of hydrocarbons with various sizes, alkanes along with some alkenes and alkynes. Crude oil is formed by the decomposition of plants and animals buried underground for millions of years due to intense heat and pressure. Crude oil cannot be used directly, different components of crude oil need to be separated using a process called Fractional Distillation. This type of fractional distillation is different and is done in large industrial scale to separate the different components of crude oil.
Firstly let’s get a clear idea about Hydrocarbons
A hydrocarbon is a compound made of carbon and hydrogen only, there are basically three types of hydrocarbons, aliphatic, alicyclic and arenes.
- Aliphatic is straight or branched chain hydrocarbons
- Alicyclic are closed ring hydrocarbons
- Arenes are hydrocarbons based on benzenes
Some common properties of hydrocarbons
Pure hydrocarbons are insoluble in water and they all burn in oxygen or air to produce carbon dioxide, water, and heat. The heat energy produced is turned to mechanical or electrical energies when we burn hydrocarbons such as gasoline in cars for example.
Hydrocarbon + Oxygen ⟶ Carbon dioxide + Water
Some sources of hydrocarbons are natural gas, coal, crude oil and fossil fuels
There are many homologous series of hydrocarbons, but let’s focus only alkane, alkene, and alkynes
General Properties of alkanes
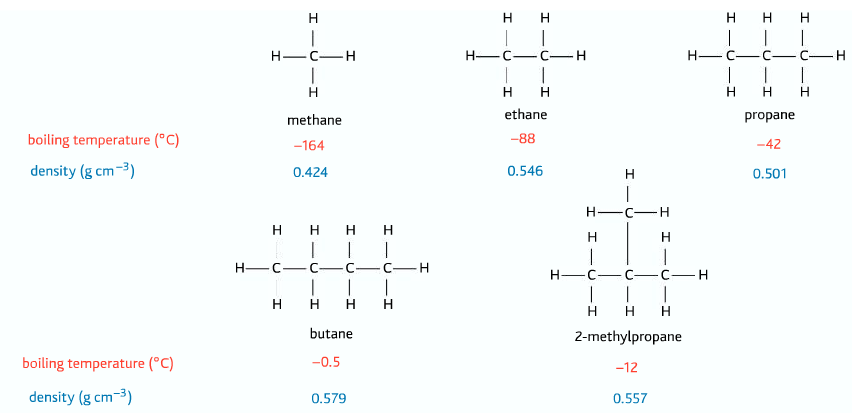
Alkanes are the simplest form of hydrocarbons, they are saturated, which means there is no carbon to carbon double or carbon to carbon triple bond present. The general formula of the alkane is CnH2n+2
As the size of the alkanes increases, their melting and boiling point increases:
How crude oil plays an important role in the economy
We depend on crude oil, we use it to run vehicles, generate electricity, use it as raw materials in the chemical industry etc. Though out demand for crude oil is massive, but the supply of crude oil is limited. Any shifts or changes in the price of crude oil affects the economy of the whole world.
How is crude oil separated
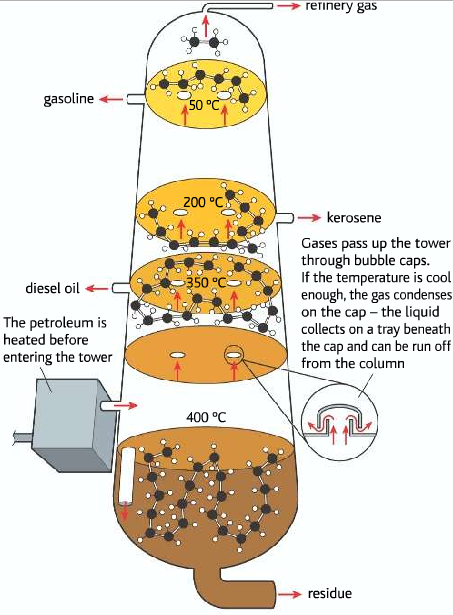
The different fractions of crude oil are separated by the process of primary distillation. The crude oil is heated and inserted into a fractionating column. Fractions of the crude oil which have a lower boiling point turn to gas moves up, while the fraction of the crude oil having higher boiling point remains at the bottom. Like this different fractions are collected at different heights of the fractionating column.
Five main fractions of crude oil: refinery gas, gasoline, kerosene, diesel oil, residue.
Five main fractions
1-2% Refinery Gas: Contains gaseous alkanes of one to four carbon atoms. Mostly contains methane used as fuel, starting point for synthesis of other organic compounds.
15-30% (Gasoline): Contains alkanes of size 5 to 10 carbon atoms Used in car engines Contains both straight and branched chains
10-15%(Kerosene): Contains alkanes made of 11 to 12 carbons Used as fuel in aircraft engines
15-20% (Diesel/ Gas oil): Used as fuel for industrial boiler, heavy types of machinery
40-50% (Residue): a Viscous mixture of hydrocarbons, high b.p, and m.p It is used for fuel for furnaces of power stations or large ships can be further fractioned to produce lubricating oils, waxes, and bitumen which is used to make roads.
The lighter fractions obtained burn more easily than heavier ones, heavier fractions are more difficult to burn. Therefore lighter hydrocarbons have higher demand. But in reality, almost half of the fractions produced are heavy ones.
Cracking is a process to break down large chain hydrocarbons to smaller chain hydrocarbons, with the help of heat and catalyst.
The cracking produces a mixture of alkanes and alkenes.
The larger fraction of hydrocarbon is converted to gas by heating, then passed over a catalyst of mixed silicon dioxide and aluminum oxide at about 600-700◦C. If catalysts are not used, then higher temperature is needed.
Cracking is a random process, so the smaller fractions produced during cracking might not be the same
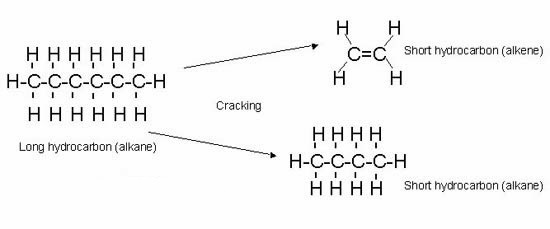
For example a large alkane C13H28 can breakdown into several combinations of alkanes and alkenes
C13H28 ⟶C2H4 + C3H6 + C8H18
C13H28⟶2C2H4 + C9H20
C13H28⟶2C2H4 + C3H6 + C6H14
C13H28⟶2C2H4 + C3H6 + C6H12 + H2
Knocking Effect in cars
When an engine in a car is running, the pistons move up and down. This up and down movement of piston provides power to the engine. The small explosion occurs inside each piston, the fuel inside each piston is artificially ignited by the spark plug at the right time.But during the knocking effect, the explosion occurs earlier.When the piston is moving down, the content inside gets hot due to compression. It gets so hot that it self-ignites. When the piston is moving down the explosion causes upward force, which decreases power obtained from the engine, damages the engine and causes a knocking sound. Petrol contains alkanes of 5-10 carbon atoms long, straight chain alkanes such as heptane, octane, and nonane ignites easily and causes knocking.
Branched-chain alkanes are more stable, doesn’t ignite easily and knocking is reduced. Branched-chain alkane such as 2,2,4-trimethylpentane is an example of branched alkane fuel, also called isooctane)2,2,4-trimethylpentane is used mixing with other fuels. The percentage of 2,2,4-trimethylpentane in a fuel is known as the octane number.
Tetraethyllead(IV) [Pb(C2H5)4] prevents early ignition. But this causes lead pollution. Artificially branched chains can be made by a process called catalytic reforming. In this process, straight-chain alkanes are broken down by the help of heat and catalyst, which again joins to form branched chain alkanes.
To clear up your concepts about Hyrdocarbons, alkane, and alkene. Please view this article IGCSE Organic Chemistry Notes

