Global Warming
Global warming is a phenomenon of heating of the earth’s atmosphere. The sun’s infrared radiation comes into the earth, goes through the atmosphere and absorbed by the earth’s surface. The earth then reradiates the infrared with a longer wavelength, that infrared radiation is absorbed by the greenhouse gases in the atmosphere. This process is called greenhouse effect, the heating up of the planet due to this effect is called global warming.
Some greenhouse gases are more effective than others at absorbing infrared radiation. To compare them a term Relative greenhouse factor is used.
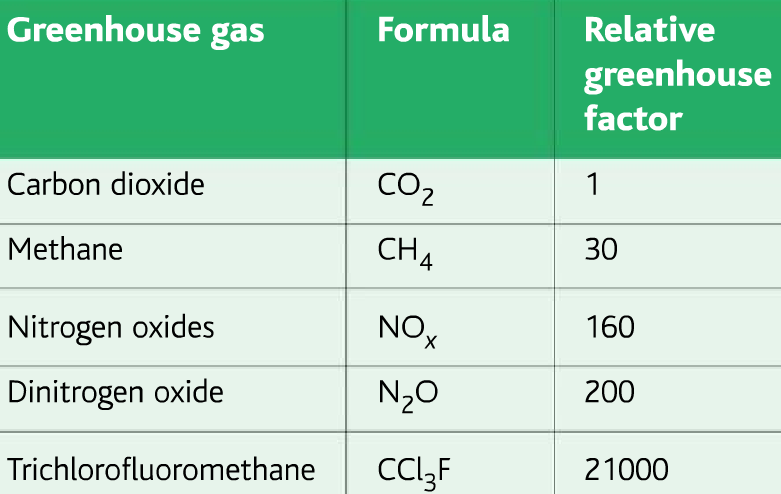
The net effect of individual greenhouse gases on global warming is determined by two properties.
- Their ability to absorb infrared radiation (Relative greenhouse factor)
- The half-life in the atmosphere, how long the gases stay.
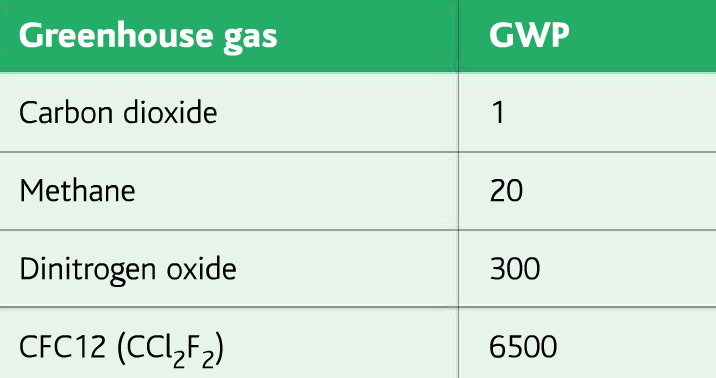
Combining these two factors gives Global Warming Potential(GWP)
Anthropogenic and natural climate change:
Anthropogenic climate change → the climate change that is due to human activities such as burning fossil fuel, deforestations etc.
Natural climate change→ Climate change due to natural processes such as carbon dioxide gas dissolving in water.
The main greenhouse gases →CO2 , NO2 , CH4
In the past hundred years, the level of greenhouse gases rose alarmingly, mainly due to human activities such as the burning of fossil fuel and deforestation.
- When a jet plane flies, it releases nitrogen oxides, which is a greenhouse gas and destroys ozone in the upper atmosphere.
Carbon neutrality
A fuel is said to be carbon neutral if it absorbs the same amount of carbon dioxide it releases when it is burned.
Example:
Trees are carbon neutral because it absorbs the same amount of carbon dioxide it releases when it is burned.
But consider the following cases
- Petroleum fuels are not carbon neutral because there is no absorption of carbon dioxide, it is simply released.
- Biofuel (ethanol by fermentation) is also not carbon neutral because CO2 is released while processing the fuel.
- Hydrogen is also not carbon neutral, you might think that burning hydrogen only releases water and no carbon dioxide. But carbon dioxide gas is released while processing the hydrogen gas.
- Even windmill is also not carbon neutral as CO2 is released while building the windmill and also the transmission lines.
Carbon footprint:
It is the amount of carbon dioxide released into the atmosphere from the activities of an individual, organization or community.
- It is measured as mass of carbon dioxide, kg or tonnes
- Suppose you buy a plastic bottle, when you throw it away it would be burned and carbon dioxide would be released. The amount of carbon dioxide released would add to your carbon footprint.
Carbon offsetting
Individuals or organization paying money to plant trees, which would absorb carbon dioxide while growing up, thus reducing their carbon footprint.
CFC and HCFC
Depletes ozone layer, as explained before. But they are also powerful greenhouse gases and contributes in global warming.
Reducing hazards and pollution in the chemical industries
Disposing of solid waste
Recycling items such as glass, metals, papers, and cardboard save earth’s natural resources, needs less energy.
Better to recycle glass than using lots of energy to make new glass.
Recycling aluminum instead of wasting so much energy to extract aluminum again by electrolysis.
Solid materials which cannot be recycled can be disposed of in two ways:
- Incineration (Combustion or burning the waste) :
Advantage: Energy recovery, while burning energy is released which can be used to produce electricity.
Disadvantage: Releases harmful gases to the environment
- Landfill:
Advantage: Doesn’t release harmful gases
Disadvantage: Uses up space, methane gas is produced from a waste product, which is a greenhouse gas. Also methane gas may cause an explosion.
Disposing of wastewater
Factories have to pay for each liter of water used. So they store the used water, treat it to use it again. The harmful substances are precipitated then filtered.
Disposing of waste gases
One of the most common waste gas is sulfur dioxide, which can be removed by using limestone.
2CaCO3 (s) + 2SO2(g) + O2(g) → 2CaSO4 (s) + 2CO2 (g)
Carbon capture: This plan includes liquefying the carbon dioxide gas and storing it under wells where natural gas used to be.
Renewable Resources:
Renewable sources are needed to be used because fossil fuels can be expensive.
Such as bio-oil from wheat, wheat can be grown again.
Ethanol from organic waste
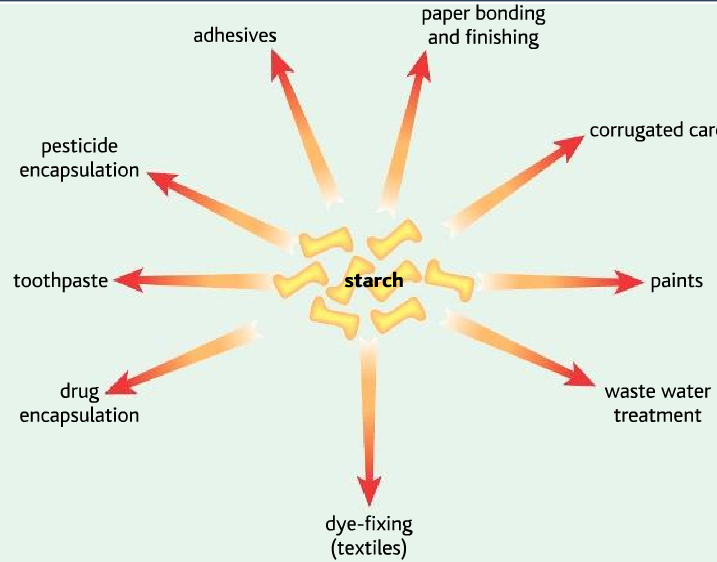
Starch from plants:
Increasing Efficiency in the chemical industry
Using a catalyst makes reaction faster at lower temperatures, thus saving energy
Atom economy: Using a reaction to produce the desired product with higher atom economy.
Example:
Reaction 1: A+B → C+D
Reaction 2: A+ E → C
Reaction 2 should be used, as the atom economy is 100% as C is the only product
Choosing a catalyst
Choosing a catalyst based on the following:
- A catalyst which works at lower temperature and pressure.
- A catalyst which is not so expensive
- A catalyst which is easy to remove from the reaction mixture
- Typical catalysts based on cobalt, rhodium, and iridium
Saving energy in the chemical industry:
In chemical industries energy comes from burning fossil fuel such as natural gas or coal:
Methods of saving energy:
- Good Housekeeping: using proper insulation to prevent heat loss
- Heat recovery: Extracting heat energy from hot waste gases
- Choice of reaction conditions: carrying out a reaction in the presence of a catalyst, so reaction occurs at lower temperature and pressure is needed. Using pure oxygen instead of air where required.
- Using microwaves in the pharmaceutical industry: Using microwaves heats up a solution in a very short time.

