IAL Chemistry is hard, really really hard. I have seen many students who got 9 straight A’s in their IGCSE exam, ended up with a C or D grade in their IAL exams. I will say that IAL chemistry is 10 times more difficult than IGCSE chemistry. Especially the unit-2 exam will absolutely blow your mind, it is that damn difficult! It is more about surviving rather than getting an excellent grade.
In this blog post, I am providing with you some really useful unit-2 common questions with answers. I have divided the questions and answers into different categories. Not only this will help you to easily digest the information better, but will also show you that some kinds of questions are indeed repeated in IAL Chemistry unit-2 exams. But the repeat questions are not 100% straightforward, they do try to make your life as difficult as possible.
With these common questions and answers in your mind, you will easily be able to get some marks in the bag in your IAL Chemistry unit 2 exam. Remember, in IAL Chemistry it’s mostly about surviving.
NOTE: For each of the categories of common question-answers I will firstly do a brief explanation, then I will go on to the actual question-answers. The categories are not arranged in any kind of order.
This blog post will be constantly updated with more common questions!
For the best experience, please view from desktop or laptop.
If your concepts with A-Level Chemistry is very weak, then you will find it very difficult to make full use of these useful common question-answers.
Reaction of sodium with alcohol questions
Observation of reaction of Sodium with water
From IGCSE chemistry I hope you know that the sodium metal reacts vigorously with water(H2O). The sodium metal will float on the surface if water and darts around as it releases hydrogen gas while it reacts with water. Here is the reaction below of the reaction between water and sodium metal.
Sodium + Water ➝ Sodium Hydroxide + Water
Na(s) + H2O(l) ➝ NaOH(aq) + H2(g)
So we can note down several key observations when sodium reacts with water.
- Sodium floats on the surface of the water
- Sodium reacts vigorously with water
- Vigorous effervescence/bubbling of hydrrogen gas
- The end product is a colorless solution
Observation of reaction of Sodium with alcohol
Alcohol just looks like water, it is colorless and you cannot tell the difference between alcohol and water when you keep them side by side. But alcohol and water do not have the same chemical and physical properties. Sodium also reacts with alcohols, but it does not react in the same way it does with water. If you do not have much idea about alcohol, then please have a quick look at my blog “IAL Alcohol organic chemistry Notes“. Sodium mainly reacts with the -OH part of the alcohol. Below is the reaction between sodium and ethanol.
Sodium + Ethanol ➝ Sodium Ethanoate + Hydrogen gas
2Na(s) + 2CH3CH2OH(l) ➝ 2CH3CH2ONa(aq) + H2(g)
From the above symbols reaction, you can see that Na simply replaces the H from the -OH group of alcohols. But in this category of common questions, we are also concerned with the observations too. Unlike the previous reaction, when sodium reacts gently with alcohol, it sinks and releases gentle effervescence of hydrogen gas.
So we can note down several key observations when sodium reacts with alcohol.
- Sodium sinks in the alcohol
- Fizzing / effervescence / bubbles (this is due to the release of hydrogen gas)
- Sodium dissolves / disappears / reduces in size
- White solid /precipitate forms ( the white precipitate is sodium ethanoate)
Related common questions regarding this topic
I going to show you a few common questions, so you will get the idea and pattern. I have taken screenshots from different parts of the question papers for these types of questions. Now you will see why I have taken the time to explain the things above.

Answer:
You will need to include only any two of the following points for this question as it is 2 marks.
– Sodium sinks into the alcohol
– Fizzing/effervescence/bubbles (this is due to the release of hydrogen gas)
– Sodium dissolves / disappears / reduces in size
– White solid /precipitate forms ( the white precipitate is sodium ethanoate)
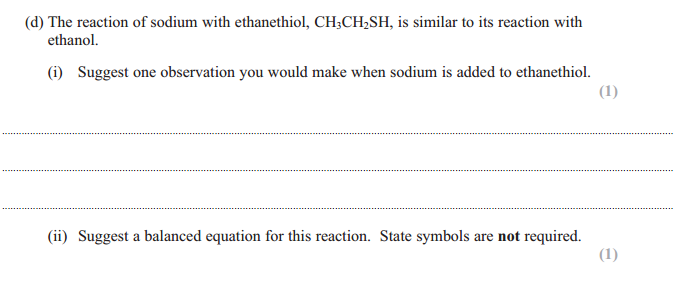
Answer for d(i) :
You will need to include only any one of the following points for this question as it is 1 mark.
– Sodium sinks into the alcohol
– Fizzing/effervescence / bubbles (this is due to the release of hydrogen gas)
– Sodium dissolves / disappears / reduces in size
– White solid /precipitate forms ( the white precipitate is sodium ethanoate)
Answer for d(ii) :
They have twisted the question a little bit, instead of an oxygen atom in the -OH group they have replaced it with a sulfur atom. But remember, both the sulfur and oxygen atom are in the same group, they have the same number of electrons in the outer shell. So just assume the reaction will go on and happen exactly the same way. The sodium atom will simply go on and replace the hydrogen atom.
Here is the balanced equation:
2Na + 2CH3CH2SH ➝ 2CH3CH2SNa + H2

Answer for b(i) :
You will need to include only any two of the following points for this question as it is 2 marks.
– Sodium sinks into the alcohol
– Fizzing/effervescence / bubbles (this is due to the release of hydrogen gas)
– Sodium dissolves / disappears / reduces in size
– White solid /precipitate forms ( the white precipitate is sodium ethanoate)
Answer for b(i) :
Methanol is CH3OH, remember, the Na will simply replace the Hydrogen atom in -OH
2Na + 2CH3OH ➝ 2CH3ONa + H2
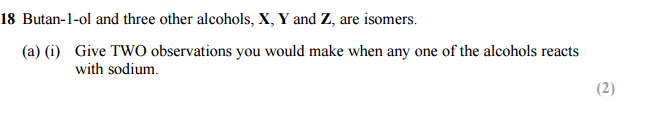
Answer for 18 a(i) :
You will need to include only any two of the following points for this question as it is 2 marks.
– Sodium sinks into the alcohol
– Fizzing/effervescence/bubbles (this is due to the release of hydrogen gas)
– Sodium dissolves / disappears / reduces in size
– White solid /precipitate forms ( the white precipitate is sodium ethanoate)
Reminder: you can get these common questions and answers Edexcel IAL Chemistry Unit 2 Common Questions and Answers: Study and Revision Guide from Amazon in kindle or paperback formats

Revise IAL Chemistry unit 2 within 1 hour & get above 90%
- No BS, to the point, quick, and easy to revise.
- Actual common questions with answers from the question paper. Get easy marks in the bag during your exam.
- Questions are divided into different topics.
- A brief discussion on each topic to get your concepts clear.
- Available in kindle ebook and paperback formats on Amazon
How Catalyst works questions
Questions related to catalysts are very common in both IGCSE chemistry and IAL chemistry. Usually, they are small 2-3 marks questions that you can easily get in the bag. I am going to show you 2-3 common questions which are going to clear up your concepts
How does a catalyst speeds up the chemical reaction? Here are the important points
- Catalyst provides an alternative route/mechanism
- That alternative route has a lower activation energy
- So a higher proportion) molecules / collisions have energy equal to or greater than the activation energy(Ea)
- A catlyst does not gets used up, so mass of catalyst at the beginning must be equal to the mass at the end

Answer for (iii) :
For the following question you will need to include three points are the question is of 3 marks. Please refer to the purple box above. Here are the three points below:
– Catalyst provides an alternative route/mechanism
– That alternative route has a lower activation energy
– So a higher proportion) molecules/collisions have energy equal to or greater than the activation energy(Ea)
To make it look even more nice and pretty, you can add the following line “so more successful collisions occur.”
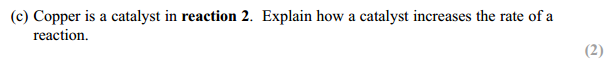
Answer for (c) :
For the following question, you will need to include only two points as the question is for 2 marks. Here are the two points below:
– Catalyst provides an alternative route/mechanism
– That alternative route has a lower activation energy

Answer for (d) :
For the following question, you will need to include only two points as the question is for 2 marks. Here are the two points below:
– Catalyst provides an alternative route/mechanism
– That alternative route has a lower activation energy

Answer for (v) :
These kinds of questions are especially in the experiment type of questions. We know that a catalyst simply increases the rate of reaction without being used up itself. In this question even though it seemed magnesium fuse increased the reaction, but in actual it got involved in the reaction itself and got used up.
So you can simply state of for these types of questions:
– It is not a catalyst because it got used up indicated by the decrease in mass.
Finding oxidation number questions
In the IAL unit-2 exam, you will find a lot of questions asking you to find the oxidation number of an element in a compound, the change in oxidation number after the reaction occurs, and finally commenting whether the element is oxidized or reduced. If your understanding page oxidation number is not clear, then please have a quick look at my blog post “IAL Redox Reactions in Chemistry Notes”. In this post, I have explained REDOX reactions and oxidation number concepts in great detail.
So if your concepts with oxidation number and redox reaction are clear, then you can easily put some marks in your bag in the unit 2 exam.
Important concepts about oxidation number (DO NOT SKIP)
- The Oxidation number of any uncombined element is zero: the oxidation number of an element is zero because it has neither gained or lost an electron, the oxidation number simply represents the change in the number of electron or electrons.
- The oxidation number of an uncombined ion is the same as its charge: for example, an uncombined ion such as Na+ will have an oxidation number of +1. Another example, an uncombined ion of O2-(Oxide ion) will have an oxidation number of -2.
- The sum of all the oxidation numbers in a molecule is zero: For example, in a carbon dioxide molecule, the sum of oxidation numbers of carbon and the oxygen would be zero. In the case of a complicated ion such as NO3–(nitrate ion), the sum of the oxidation number would be equal to the charge of the complicated ion, in this case -1. Oxidation numbers in covalent compounds would be discussed in details in the latter part of this article
- Fluorine always has an oxidation number of -1: This statement means that whenever fluorine undergoes a reaction, it always ends up with an oxidation number of -1. This concept would be cleared up when we will see some examples of how to calculate the oxidation number.
- Hydrogen always has an oxidation number of +1, except in metal hydrides (in those cases the oxidation number is -1)
- When oxygen forms a compound it always ends up with an oxidation number of -2: some exceptions are when oxygen is in peroxides such as H2O2(Hydrogen Peroxide, in this case, the oxidation number of oxygen is -1), also when oxygen combines with fluorine it ends up with a positive oxidation number!
- Chlorine always ends up with an oxidation number of -1 when it is a compound: one exception is when chlorine forms a compound with fluorine. In that case, chlorine ends with a positive oxidation number. This is because fluorine is more electronegative than chlorine.
- Group 1 elements always ends up with an oxidation number of +1 when they form compounds, group 2 ends up with +2 and group 3 ends up with +3: for example, Na in group 1 becomes Na+ which has an oxidation number of +1, Ca in group 3 becomes Ca+2 which has an oxidation number of +2 and Al in group 3 becomes Al+3 which has an oxidation number of +3.
What is the oxidation number of chlorine in Cl2O7?
Answer:
The overall sum of oxidation numbers in the molecule is 0 as the molecule does not have any charge.
Let the oxidation number of chlorine in the molecule be y, when oxygen is combined with other elements it has an oxidation number of -2.
So we can write:
2y + (7x-2) = 0
2y-14 = 0
2y = 14
y = 14/2 = 7
Hence the oxidation number of chlorine in this compound in +7
The equation for the reaction between iodide ions and iodine monochloride is given below. Show that this is a redox reaction by giving all the oxidation numbers and identifying the oxidizing agent. (2)
ICl + I – → I2 + Cl–
Oxidation numbers ……… ……… ……… ………
Oxidizing agent:
Answer:
Oxidation Numbers
ICl : Oxidation number of Iodine= -1, Oxidation number of Chlorine = -1
I- : Oxidation number of Iodine = -1
I2: Oxidation number of iodine = 0
Cl-: oxidation number of Chlorine= -1
Oxidation Agent: monochloride, because it oxidizes the Iodide ions to Iodine, oxidation number increases from -1 to 0
When iodide ions react with concentrated sulfuric acid, another product, X, can also be detected. X is a toxic gas with the smell of rotten eggs. Identify X, by name or formula, and give the oxidation numbers of sulfur when X is formed from concentrated sulfuric acid. (3)
Answer:
The gas is Hydrogen Sulfide (H2S), because hydrogen sulfide gas smells like rotten eggs.
The oxidation number of sulfur in X (Hydrogen Sulfide):
We know the oxidation number of hydrogen is +1 when combined (please refer to the box above)
The sum of oxidation numbers in the molecule is 0, as there is no overall charge.
(2×1) + S = 0
2+S = 0
S= -2
Hence the oxidation number of sulfur in hydrogen sulfide is -2
The oxidation number of sulfur in sulfuric acid:
Formula of sulfuric acid: H2SO4
We know the oxidation number of hydrogen is +1 when combined, the oxidation number of oxygen is -2 when combined.
(2 x 1)+ S + (4 x -2) = 0
2 + S – 8 =0
S-6 = 0
S = +6
Hence the oxidation number of sulfur is sulfuric acid is +6
The nitrogen atoms in ammonia are oxidized. Give the oxidation numbers of the nitrogen atoms in ammonia and nitrogen monoxide.
Answer:
Formula of Ammonia: NH3
When combined, Hydrogen have an oxidation number of +1
N + (3 x 1) = 0
N + 3 = 0
N = -3
Hence the oxidation number of nitrogen in ammonia is -3
The formula of Nitrogen monoxide: NO
When combined oxygen have an oxidation number of -2
N -2 = 0
N = +2
Hence Oxidation number of nitrogen in nitrogen monoxide is -2
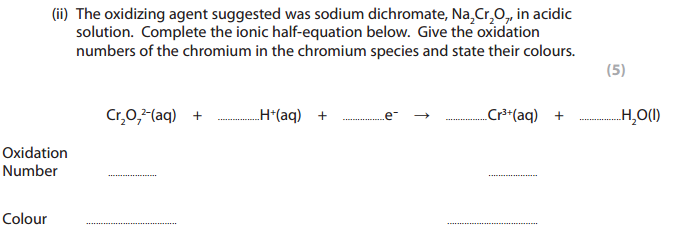
Answer for (ii) :
Ionic Half Equation: Cr2O72- + 14H+ + 6e– ➝ 2Cr3+ + 7H2O
Oxidation number of Chromium in Cr2O72-:
(2 x Cr) + (7 x -2) = -2
2Cr – 14 = -2
2Cr = 14 -2
2Cr = 12
Cr = 12/2 = +6
On the right-hand side, the oxidation number of chromium is +3, because it is an uncombined ion with a charge of +3, so the oxidation number is the same as the charge.
Color of Cr2O72-solution: orange
Color of Cr3+ solution: green
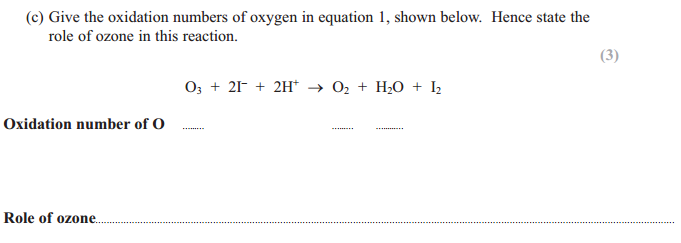
Answer for (c) :
The oxidation number of O in Ozone or O3 is 0 (zero) because it is an element
The oxidation number of O in O2 is 0 (zero) because it is an element
The oxidation number of O in H2O is -2 because when combined, oxygen has an oxidation number of -2
Role of Ozone: ozone increases the oxidation number of iodine from -1 in iodide ions to 0 in iodine. Hence it is acting as an oxidizing agent over here.
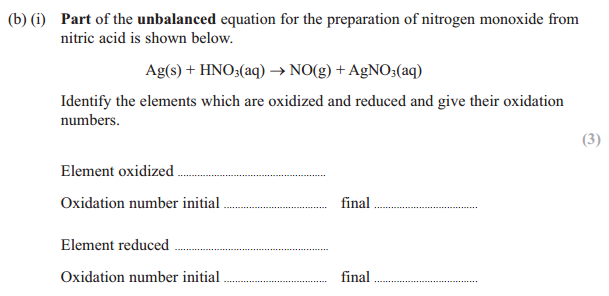
Answer for (b)(i) :
Silver(Ag) is oxidized
Oxidation number increases from 0 in Ag to +1 in AgNO3
Nitrogen is reduced,
Oxidation number of decreases from +5 in HNO3 to +2 in NO
Finding oxidation number of nitrogen is HNO3:
+1 + N + (-2 x 3) = 0
+1 + N + -6 = 0
N-5 = 0
N = +5
Finding oxidation number of nitrogen in NO:
N -2 = 0
N = +2
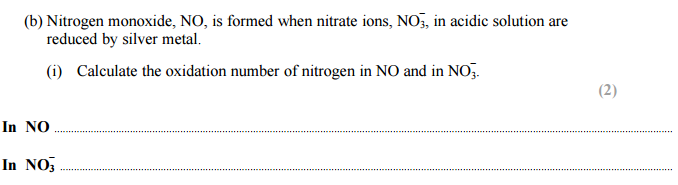
Answer for (b) :
In NO: +2
In NO3- ; +5
Finding oxidation number of nitrogen is NO3–:
N + (-2 x 3) = -1
N -6 = -1
N = 6-1
N = +5
Finding oxidation number of nitrogen in NO:
N -2 = 0
N = +2
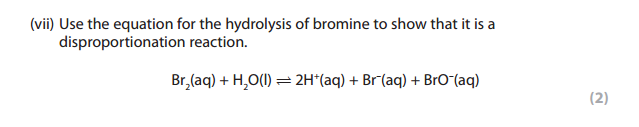
The disproportionation reaction is a reaction in which the same element is both oxidized and reduced.
We can see that bromine goes from Br2 to Br– and BrO–
The oxidation number of bromine in Br2 = 0
The oxidation number of bromine in Br– = -1
The oxidation number of bromine in BrO- :
Br -2 = -1
Br = 2 -1 = +1
Answer: Bromine is both oxidized and reduced. Oxidized because its oxidation number increases from 0 in Br2 to +1 in BrO-.
Reduced because its oxidation number decreases from 0 in Br2 to -1 in Br–. Hence this is a disproportionation reaction.
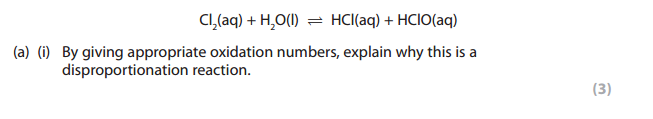
We can see that in the reaction above Cl2 becomes HCl and HClO
Oxidation number of chlorine in Cl2 = 0
Oxidation number of chlorine in HCl = -1
Oxidation number of chlorine in HClO:
1 + Cl -2 = 0
Cl -1 = 0
Cl = +1
Answer: the following above is a disproportionation reaction because in this reaction chlorine is both oxidized and reduced.
Oxidized because the oxidation number of chlorine increases from 0 in Cl2 to +1 in HClO
Reduced because the oxidation number of chlorine decreases from 0 in Cl2 to -1 in HCl

Oxidation number of Iodine in HIO3:
1 + I + (-2 x 3) = 0
I -5 = 0
I = +5
Oxidation number of Iodine in I2O5:
(2 x I) + (-2 x 5) = 0
2I -10 = 0
2I = 10
I = +5
Answer: No, this is not a REDOX reaction, because the oxidation number of Iodine remains the same.
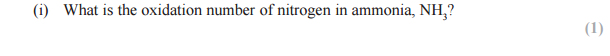
Answer:
N + (3 x 1) = 0
N +3 = 0
N = -3
The following two screenshots below are from the same question. The left part is the first part while the right part is the second part. In this example, I am going to directly state the oxidation numbers, because by now after all these examples you should know how to find the oxidation number. You can click on the screenshots below to enlarge them.
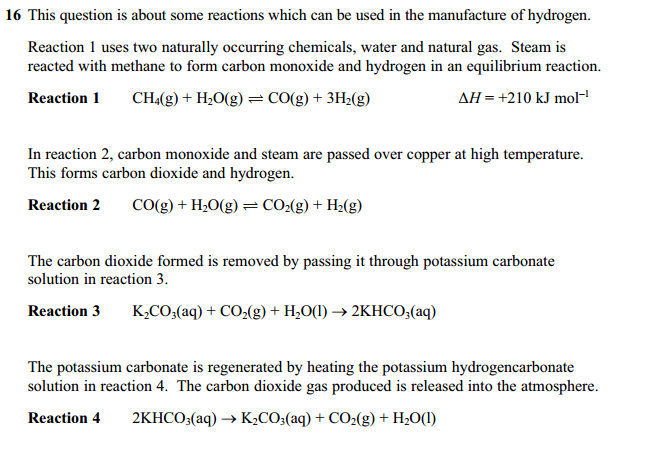

Reaction 1: The oxidation number of carbon increases from -4 in CH4 to +2 in CO, hence it is oxidized. The oxidation number of hydrogen decreases from +1 in H2O to 0 in H2, hence it is reduced. As both oxidation and reduction occur in the same reaction, it is a REDOX reaction.
Reaction 2: The oxidation number of carbon increases from +2 in CO to +4 in CO2, hence it is oxidized. The oxidation number of hydrogen decreases from +1 in H2O to 0 in H2, hence it is reduced. As both oxidation and reduction occur in the same reaction, it is a REDOX reaction.
Reaction 3: The oxidation number of carbon is +4 on both sides, Hydrogen +1 on both sides, potassium also +1 on both sides of the reaction. As all of the oxidation numbers of all of the elements are constant, none of the elements are oxidized or reduced. Hence the following reaction is NOT a REDOX reaction.
Reminder: you can get these common questions and answers Edexcel IAL Chemistry Unit 2 Common Questions and Answers: Study and Revision Guide from Amazon in kindle or paperback formats

Revise IAL Chemistry unit 2 within 1 hour & get above 90%
- No BS, to the point, quick, and easy to revise.
- Actual common questions with answers from the question paper. Get easy marks in the bag during your exam.
- Questions are divided into different topics.
- A brief discussion on each topic to get your concepts clear.
- Available in kindle ebook and paperback formats on Amazon
Flame test questions
In IAL Chemistry unit 2 exam you will find some questions related to the flame test. You should keep in mind the following things in order to answer the flame test-related questions.
- Colors for different cations after the flame test is performed.
- How to perform the flame test.
- The reason for that specific color or no color for the result of the flame test.
How to perform a flame test
How to perform the flame test:
Add a few drops of concentrated hydrochloric acid to the sample in a watch glass. Then dip a clean platinum or nichrome wite into the mixture. Then hold the wire in the flame of the bunsen burner. You will be able to see a color or no color, depending on the ion present.
Result colors for different ions after the flame test is performed
| Ion | Color |
| Lithium | Red |
| Sodium | Yellow |
| Potassium | Lilac |
| Magnesium | No color |
| Calcium | Yellow red |
| Strontium | red |
| Barium | Pale green |
Reason for flame color after the flame test is performed
When we perform the flame test, we may say a color (not in the case of magnesium). We see the flame colors due to the reasons listed down below.
- Electrons are promoted/ jump / become excited to higher energy level
- Electron(s) return/ fall back to lower energy level
- When electrons fall back to lower energy level they release of photons
The chlorides of calcium and barium can be distinguished using flame tests. State what you would see in each test. (2)
Answer:
We can see a yellow-red color for calcium chloride.
We can see a pale green color for barium chloride.
The flame test on a magnesium salt, such as magnesium chloride, produces no color. Describe the electronic transitions involved in a flame test and suggest why there is no flame color for magnesium chloride.
Answer:
During the flame test, the electrons are promoted to higher energy levels. When they fall back to lower energy levels, they release photons. In the case of Magnesium ions, the photons released do not fall in the visible spectrum hence we cannot see any flame color.
A flame test was carried out on a solid calcium compound. Explain the origin of the flame color in terms of electron movement.
Answer:
During the flame test, the electrons are promoted to higher energy levels. When they fall back to a lower energy level, they release photons.
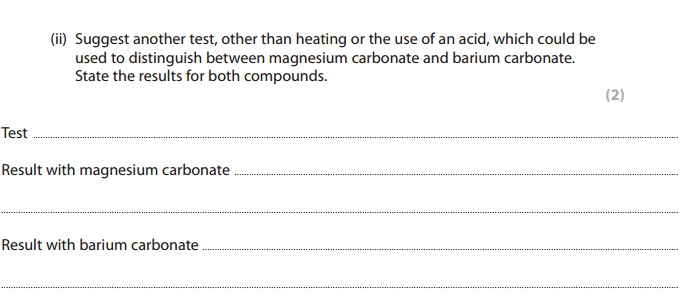
Test: Flame test
Result with Magnesium Carbonate: No flame color is seen
Result with Barium Carbonate: pale green flame color is seen

Magnesium Carbonate: No flame color is seen
Calcium Carbonate: Yellow red flame color is seen

Color: Lilac
Stability of Group 1 and 2 Nitrates Questions
I hope by now at this stage you are familiar with group 1 and 2 metals in the periodic tables. Examples of some group 1 metals are potassium, sodium, lithium, etc. Some examples of group 2 metals are magnesium, calcium, barium, etc.
Some examples of group 1 nitrates are sodium nitrate(NaNO3), potassium nitrate (NaNO3), lithium nitrate (LiNO3).
Some examples of group 2 and other kinds of nitrates are magnesium nitrate(Mg(NO3)2), calcium nitrate(Ca(NO3)2), copper(II)nitrate (Cu(NO3)2), etc.
We can divide the nitrates or metal nitrates into two types, group 1 nitrates, and other metal nitrates. Group 1 nitrates decompose in a different way when they are heated. This is because all of their ions have a +1 charge and lower polarizing power. Please have a look at the box below to understand the important points.
Decomposition of group 1 nitrates vs other nitrates when heated.
Group 1 metal nitrates except lithium nitrate decompose on heating to produce metal nitrite and oxygen gas
For Example:
Potassium Nitrate ➝ Potassium Nitrite + Oxygen
2KNO3 ➝ 2KNO2 + O2
All other metal nitrates decompose on heating to produce metal oxide, nitrogen dioxide, and oxygen gas.
For Example:
Magnesium nitrate ➝ Magnesium Oxide + Nitrogen dioxide + Oxygen
2Mg(NO3)2 ➝ 2MgO + 4NO2 + O2
Lithium nitrate acts like group 2 nitrates because even though lithium-ion has a +1 charge, as it is very small with a lower surface area are makes it highly polarizing.
Decomposition of lithium nitrate on heating:
Lithium nitrate ➝ Lithium Oxide + Nitrogen dioxide + Oxygen
4LiNO3 ➝ 2Li2O + 4NO2 + O2
The nitrogen dioxide gas is brown in color. In the case of group 1 nitrates, we will see no visible changes as the oxygen gas released is colorless. But in the case of decomposition of other nitrates such as calcium nitrate, barium nitrate, etc. we can visibly see the formation of brown nitrogen gas.
The stability of group nitrates increases down the group because:
- Down the group the size of group 2 cation decreases.
- Hence they polarize the nitrate ions less.
Here are the common question answers related to group 1 and 2 nitrates
Hydrated barium nitrate, Ba(NO3)2.4H2O, is strongly heated in a boiling tube and the following changes occur.
Stage 1 The white solid forms a clear colorless solution.
Stage 2 Condensation forms around the mouth of the boiling tube and a white solid starts to form at the bottom of the tube.
Stage 3 As the heating continues, the colorless solution disappears leaving another white solid.
Stage 4 This white solid melts.
Stage 5 Nitrogen dioxide and oxygen gases are given off, and barium oxide is left in the test tube.
(a) (i) Give the formula for the white solid formed in Stage 3. (1)
Ans: Ba(NO3)2
(ii) What would you see when nitrogen dioxide is given off in Stage 5? (1)
Ans: (Nitrogen dioxide is a) brown gas/fumes/vapour
(i) Describe the test for oxygen and its positive result.(1)
Ans: Oxygen relights/rekindles a glowing splint
(ii) Describe a simple test-tube experiment that you can use to compare the thermal stabilities of anhydrous barium nitrate and anhydrous calcium nitrate. State two essential conditions necessary to ensure a fair test. You may wish to draw a diagram. Detailed measurements are not required.
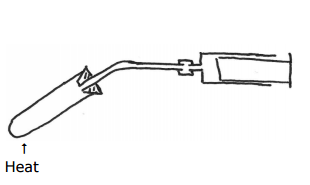
- Take two separate test tube on containing barium nitrate and the other containing calcium nitrate. Then apply heat by arranging the apparatus as in the right.
- Make sure you take in the respective mass of each containing equal number of moles.
- Identical heating /same amount of heat /constant heating
*(c) Explain why anhydrous calcium nitrate decomposes more readily than anhydrous barium nitrate.(3)
- Calcium (ions) are smaller than barium (ions) /have a higher charge density
- The calcium ion polarizes/distorts
- The nitrate/anion (ion)/N-O bond is polarised/distorted/broken (this weakens the N-O bond)
(f) Magnesium nitrate is another salt of magnesium. It decomposes when heated to produce nitrogen dioxide and oxygen. Balance the following equation. State symbols are not required. (1)
…………………Mg(NO3)2 ➝ …………………MgO + …………………NO2 + …………………O2
Answer: Here is the balanced equation below:
2Mg(NO3)2 ➝ 2MgO + 4NO2 + O2
Identify two ways, one of which should be an observation, in which the thermal decomposition of anhydrous calcium nitrate is different from that of anhydrous potassium nitrate.
- • (Calcium nitrate) produces a brown/ red-brown gas
- • (Potassium nitrate) does not produce a brown gas

i) 2KNO3 → 2KNO2 + O2
ii) ii) 2Ca(NO3)2 → 2CaO + 4NO2 + O2

- Formation of brown gas (which is nitrogen dioxide)
- The solid melts

- Calcium ions have greater positive charge (than potassium ions)
- Calcium (ions) more polarising or cause greater distortion than the potassium ions
- Hence it poloarises the nitrate ion more

2Mg(NO3)2 → 2MgO + 4NO2 + O2

Magnesium ions are smaller than calcium ions, hence it polarizes the nitrate ion more

i) 2NaNO3 → 2NaNO2 + O2
Reminder: you can get these common questions and answers Edexcel IAL Chemistry Unit 2 Common Questions and Answers: Study and Revision Guide from Amazon in kindle or paperback formats

Revise IAL Chemistry unit 2 within 1 hour & get above 90%
- No BS, to the point, quick, and easy to revise.
- Actual common questions with answers from the question paper. Get easy marks in the bag during your exam.
- Questions are divided into different topics.
- A brief discussion on each topic to get your concepts clear.
- Available in kindle ebook and paperback formats on Amazon
Stability group 1 and other carbonates Questions
You will find quite a lot of questions related to the stability of metal carbonates, what products they produce when they decompose when heated. Usually, these questions are quite straightforward and are easy marks in the unit 2 exam. But you will need to know some important points to answer these questions. Please have a look at the box below.
Some important facts about metal carbonates which will help answer the common questions
- Group 1 metal carbonates(except lithium carbonate) do not decompose on heating
- All other metal carbonates decomposes on heating to produce metal oxide and carbon dioxide gas
Metal Carbonate ➝ Metal Oxide + Carbon dioxide
MgCO3 ➝ MgO + CO2
Li2CO3 ➝ Li2O + CO2 - Stability of the metal carbonates increases down the group, because down the group, because the size of the cation increases, polarizes the carbonate ion less
- The carbon dioxide produced can be tested using limewater (Ca(OH)2) solution. Carbond dioxide turns limewater milky.

Answer:
- Barium carbonate is more thermally stable (than magnesium carbonate) / requires more heating / needs a higher temperature / decomposes more slowly / produces carbon dioxide more slowly
- MgCO3 ➝ MgO + CO2

- Take the mass which corresponds to equal number magnesium carbonate and calcium carbonate
- Heat each one with equal flame strength
- Pass the gas produced through limewater and record the time for it to go milky
- Pass the gas produced through limewater and record the time for it to go milky

- Calcium carbonate is more stable than magnesium carbonate
- Mg2+ ions are smaller than Ca2+ ions
- Hence it polarizes the the carbonate ion pore

Answer: Time for the first (permanent) cloudiness to appear in the limewater
- Constant Bunsen flame/electrical heater setting
- Fixed volume/amount/mass of limewater
- Same surface area/particle size (of solid)

Answer: More stable/(thermal stability) increases (as the group is descended)

- Cation/positive ion becomes larger down the group
- Polarisation/distortion of the carbonate ion is reduced
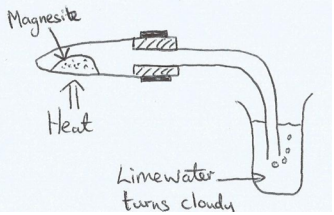
Draw a labeled diagram of the experimental apparatus that you could use to form magnesium oxide from the mineral Magnesite (magnesium carbonate). Include in the diagram how you would test for any other product of the reaction. State the expected observation from the test.
Answer:
Greenhouse gas and environment Questions
There is a specific part in unit 2 chemistry called the “Green Chemistry”. It is not only there for your knowledge, there are a lot of common green chemistry-related questions which appear in the unit 2 exams. So I am listing down all of these questions along with answers. Hope you will find them extremely helpful.
Some important points about green chemistry which will help you answer the questions:
- Green house gases are the gases which absorbs the infrared radiation re-radiated from the earth’s surface.
- Some major greenhouse gases are: water vapor (H2O), Carbon dioxide (CO2), Methane (CH4), Nitrous oxide (N2O), Ozone (O3), Chlorofluorocarbons (CFCs), Hydrofluorocarbons (includes HCFCs and HFCs)
- Carbon dioxide is a major greenhouse gas and has the most effect. Carbon dioxide is produced by burning fossil fuels in vehicle, factories, burning coals etc.
- Global warming happens both naturally and due to human activities. Anthropogenic climate change is caused by human activities such as burning fossil fuels. Natural climate change is caused by volcanic eruptions, water vapor in the air etc
- Water vapor is a green house gas but it is not considered in the anthropogenic climate change as the water vapor remained approximately constant for the previous centuries.
- Greenhouse gases such as carbon dioxide and water absorb infrared radiation by changing their bond polarities if their polar bonds.
- UV rays are harmful, it causes skin cancer as it is energy of high frequency.
- For others see the common question answers below.
Carbon dioxide is a molecule in the atmosphere that absorbs infrared radiation. Explain why this molecule absorbs infrared radiation and what effect this absorption has on the molecule.
Answer: It has polar bonds, change in (bond/molecule) polarity
Suggest a gas, of which there is a significant concentration in the atmosphere, which does not absorb infrared radiation.
Answer: Nitrogen/N2 /Oxygen/O2 / Argon/Ar
Suggest why there is now little concern over the contribution of CFCs to global warming compared with that of carbon dioxide.
Answer: (CFCs) No longer being released in the atmosphere/ less used/concentration decreasing/ amount reduced
Water vapor is another molecule in the atmosphere that absorbs infrared radiation, but it is not considered to be responsible for anthropogenic climate change. Justify this statement.
Answer: Water vapor is always present naturally, The levels of water vapor have kept relatively constant (over the recent centuries)
Explain the term ‘carbon neutrality‘ and suggest why biofuels are unlikely to be completely carbon neutral.
Answer: Carbon neutrality is where the CO2 released on combustion is equal to the CO2 absorbed on the formation of the fuel/plant, CO2 (from fossil fuels) is likely to be released from transport/production of biofuel/production of fertilizer/processing of the biofuel

Answer: bonds change polarity

Answer: Infrared radiation/heat is absorbed by greenhouse gases / by carbon dioxide and water, When energy from the sun is (re-)emitted from the earth’s surface (allow ‘reflected’).

Answer: Anthropogenic climate change is caused by human activity, Natural climate change is caused by volcanic eruptions, etc
Water vapor levels are always relatively constant, CO2 levels increasing due to (fossil) fuel combustion

Answer: (Cl radicals) break down ozone (layer)/ ozone depletion / ozone (layer) thinnings

Answer: CO2 has polar bonds/oxygen does not have polar bonds, the polarity of CO2 changes
Answer: (A greenhouse gas) traps/absorbs/ reflects IR (radiation) / heat (re-radiating) from the earth

Answer: (water is a greenhouse gas) because it absorbs infrared (IR) radiation
The polarity of the water molecule changes when its bonds vibrate
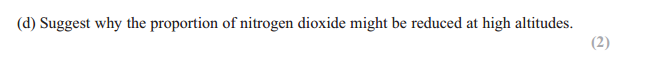
Answer: intensity of UV (radiation/light) is greater
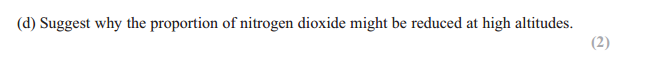
Answer: Ozone absorbs/blocks/filters/ protects UV
UV causes (skin) cancer

Answer: Aircraft (release NO) closer to the ozone layer
So less NO is lost through competing / other reactions

Answer: Sulfur dioxide/SO2 (1)
Causes acid rain (1)

Answer: Jet airplanes fly (much) close(r)/near(er) to the ozone (layer), NO from planes does not react before it can react with the ozone


Answer: Carbon dioxide
Traps IR emitted from the earth

Answer: IR /infrared radiation
Contains polar bonds which can change polarity
The sulfur dioxide combines with water and oxygen in the atmosphere to produce sulfuric acid, H2SO4. Write a balanced equation, including state symbols, for this overall reaction.
Ans: 2SO2(g) + 2H2O(l) + O2(g) → 2H2SO4(aq)
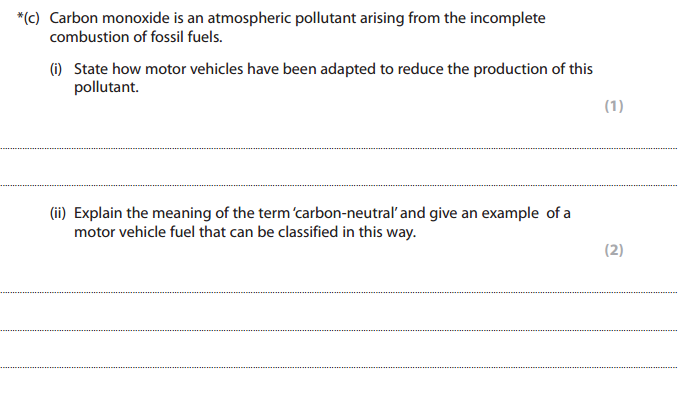
C i) (cars have a) Catalytic converter
ii) The amount of CO2 produced (on combustion) is equal to the amount of CO2 absorbed (during photosynthesis), Biofuel
State one harmful consequence to a person of increased exposure to ultraviolet radiation.
Answer: (Increased risk of) skin cancer
What property of ultraviolet radiation makes it more harmful than infrared radiation to living organisms? Justify your answer.
Answer: (UV) is high(er) energy /high(er) frequency /short(er) wavelength
Suggest why the release of free radical nitrogen oxides by vehicles, such as buses and lorries, does not affect the ozone layer.
Answer: They breakdown/react/dissolve (in the lower atmosphere before they rise to the ozone layer)
State one other environmental problem associated with coal-fired power stations. Identify the substance which causes this problem.
Answer: Global warming, carbon dioxide
Suggest two measures, other than the use of biofuels, by which the chemical industry could reduce its carbon footprint.
Answer:
Pick any two from the bottom:
Use catalysts/enzymes (to reduce energy consumption) (1)
Use microwave energy (which is more efficient) (1)
Improve thermal insulation (1)
Use heat exchangers/heat recovery (1)
Reduce waste/recycle (bi-)products (1)
Use renewable resources in its processes (1)
Use high atom economy processes (1)
Use nuclear power/renewable energy sources/use wind power/use solar power/use fuel cells (1)
Use carbon capture and storage methods (1)
Intermolecular Forces Questions
Lots of questions related to intermolecular forces appear in the IAL Chemistry unit 2 examinations. The questions are quite straightforward if you manage to remember the important points. I am not going to explain everything from scratch, just note down a few points in the box below. If your concepts about intermolecular forces are not clear, then please have a quick look at my blog post “IAL Intermolecular Forces Notes“. After mentioning the important points, I will go straight into the intermolecular forces common question answers.
Different types of intermolecular forces
- There are three types of intermolecular forces:
1)London Forces/ Dispersion forces/van der Waals’ forces
2) Dipole-Dipole interactions
3) Hydrogen bonding - Order from strongest to weakest: Hydrogen bonding > Dipole dipole interactions > dispersion forces
- Hydrogen bonding will only occur if a molecule contains if a molecule contain any one of the following bonds: H-F, O-H, N-H. Examples of molecules which form hydrogen bonding: water (H2O), Ammonia (NH3), HF.
- In a substance which has hydrogen bonding such as water will have all three of the intermolecular forces: dispersion, dipole dipole interactions and Hydrogen bonding. But the hydrogen bonding will be the most predominant.
- Substance which has dipole dipole interactions such as HCl, will have dipole diple interactions and dispersion forces present. The dipole dipole forces will be more predominant.
- Substances which doesn’t contain any polar molecules, will have dispersion forces only. The usual examples are hydrogen, carbon dioxide etc.
- How dispersion forces are formed: instantaneous imbalance in electron density causes an dipole in the adjacent molecule, which causes attraction.
- Molecules with more mass have more electrons, so the effect of instantaneous dipole will be more, hence leading to more dispersion forces, which will need more energy to separate. For example, substances which contains molecules with more mass have a greater melting/boiling points than substances which contains molecules with lower mass. But given that they do not form any strong intermolecular forces such as dipole dipole interactions or hydrogen bonding.
- Branching in organic molecules decreases the surface area which decreases the dispersion forces.
When chlorine is passed over iodine crystals, iodine monochloride, ICl, is formed. Iodine monochloride, ICl, is a liquid at room temperature whereas chlorine, Cl2, is a gas. Explain, in terms of intermolecular forces, why this is so
Answer: ICl is a polar molecule, hence iodine monochloride has dipole dipole interactions. On the other hand, chlorine molecules are non-polar and have only London forces present. Dipole dipole interactions are stronger than London forces, hence requires more energy to overcome. Hence chlorine is a gas a room temperature which iodine monochloride is a liquid.
Describe how London forces are formed
Answer: Firstly instantaneous dipole is formed in the molecule due to sudden and random imbalance of electron density, which causes induced dipole in the adjacent molecule, which leads to attraction.

Answer:
Intermolecular Forces in alkanes: dispersion forces
How they rise: they rise due to instantaneous dipole

Predominant intermolecular forces in alcohols: hydrogen bonding
How they rise: oxygen’s higher electronegativity creates dipole/large difference in electronegativity, which causes attraction between a lone pair of oxygen atoms and a partially positively charged hydrogen atom.

Answer: Alkanes have only weak dispersion forces between molecules, which takes very low energy to overcome.

Answer: There are only weak dispersion forces between fluorine molecules, on the other hand in CH3F, there are also stronger dipole dipole interactions present, which takes more energy to overcome.

Answer: Strong hydrogen bonding is present between HF molecules, which takes high energy to overcome.

Answer: carbon dioxide molecule has more electrons than methane

Answer: Butane has a greater surface area, so more contact between (neighboring) molecules / (neighboring) molecules pack better.

Answer:
Ammonia has hydrogen bonds (as well as London forces)
Methane (only) has London
Intermolecular forces in ammonia are stronger than those in methane
(Ammonia has hydrogen bonds) because nitrogen is very electronegative
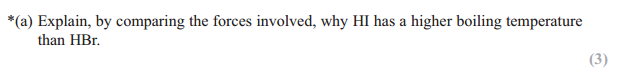
Answer:
London/dispersion forces greater in HI
Because (Iodine/HI) has more electrons
(So) more energy needed to separate molecules

Answer:
HF has hydrogen bonding (and HCl does not)
HF has hydrogen bonding (and HCl does not)
Hydrogen bonding is (much) stronger

Answer:
Water forms (up to) two hydrogen bonds

Answer:
Instantaneous dipole which causes induced dipole in an adjacent molecule

Answer:
Ethanethiol/sulfur has more electrons (so forces are stronger)


Answer:
London (forces)
dipole-dipole (forces)

Answer:
(iii) Dispersion forces
(iv) Hydrogen bonding
Reminder: you can get these common questions and answers Edexcel IAL Chemistry Unit 2 Common Questions and Answers: Study and Revision Guide from Amazon in kindle or paperback formats
Iodine starch test questions
The presence of iodine molecules in a solution can be tested using the starch solution. I am going to include only two questions for this topic as the concept is quite straightforward. Please have a look at the colored box below regarding a few key concepts about iodine and starch solution.
- In a solution where iodine molecules are present, that solution will have a pale yellow or straw color.
- When starch solution is added to a solution which contains iodine molecule, the starch molecules combine with the iodine molecules to form a blue black solution.
- In a titration reaction, if the iodine molecules are used up in the end, the blue-black color will disappear.
- Starch is added just at the end of titation reaction, becuase if it is added too soon it traps the iodine molecules.
The starch solution is usually added as an indicator towards the end of the titration. Describe how the color of the mixture would change during the titration before starch is added. (1)
Answer: pale yellow/ straw-colored
Explain why the starch solution is not added at the start of the titration. (1)
Answer: An insoluble compound forms (if starch is added too soon)

Answer:
Color before adding starch: pale yellow/straw-colored
Color after adding the starch: Blue-black
The color at the end-point: colorless
Maxwell-Boltzman distribution questions
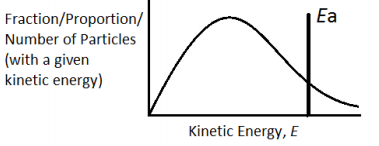
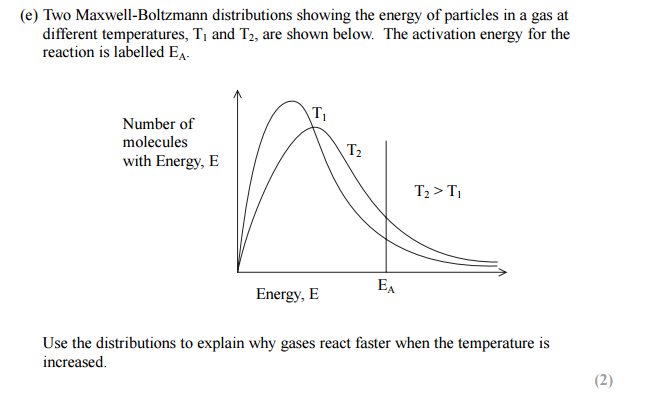
In simple words, Maxwell-Boltzman distribution gives us an idea about the distribution of kinetic energy of particles in a gas. The Maxwell-Boltzman distribution is represented by a simple graph, which shows the relationship between the number of particles and kinetic energy. Please have a look at the graph below.
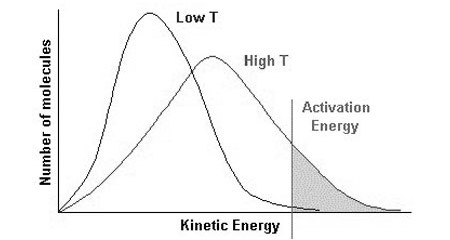
The line on the right-hand side is marked as the activation energy. The grey area represents the number of particles that have energy equal to or more than the activation energy. Particles which has energy equal to or more than the activation energy will collide and a reaction will occur, this is called a successful reaction. But the particles which have energy less than the activation energy will collide but no reaction will occur, they will simply bounce off of each other.
You can see two curves, one marked with Low T, which means low temperature, the other is marked High T, meaning higher temperature.
The peak in each curve represents the common kinetic energy that the majority number of gas particles have.
When the temperature is increased, the peak shifts to the right and becomes a little bit flat. This means the common energy which majority of particles have is more. It becomes flattered means that the majority of particles having that higher energy is less.
Another thing, when the temperature is increased, the tail of the curve on the right-hand side raises up, thus increasing the grey area. Greater grey area means more number of particles have energy equal to or more than the activation energy. As more particles have energy equal to or more than the activation energy, the more successful collision occurs.
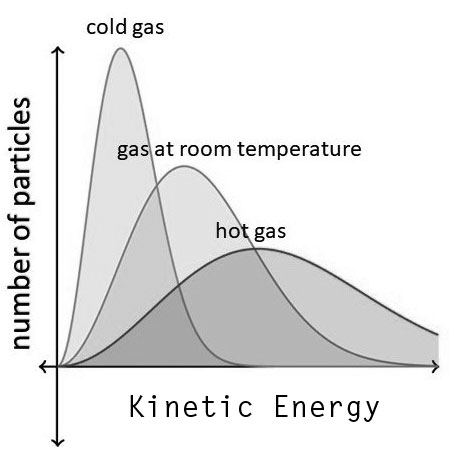
If we keep increasing the temperature, the peak will keep shifting to the right and the tail will keep rising up, thus increasing the total number of particles having energy equal to or more than the activation energy. Please have a look at the three curves below to get a better understanding of how the distribution is affected by an increase in temperature.
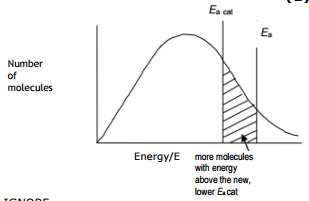
Effect of catalyst shown in Maxwell-Boltzman distribution
The catalyst provides an alternative path for the reaction which has lower activation energy. More number of particles have energy equal to or more than the activation energy, more number of successful collisions occur, and the rate of reaction increases. Please have look at the simple graph below which shows the effect of a catalyst in the Maxwell-Boltzman distribution graph.
Related Common Questions
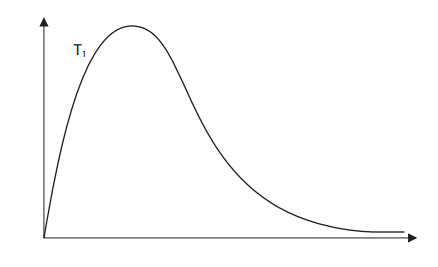
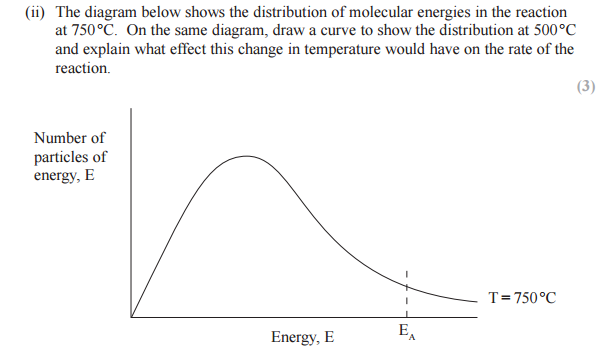
On the diagram below, draw a curve to show the distribution of molecular energies at a higher temperature, T2.
Use your diagram, with further labeling as necessary, to explain why the rate of a chemical reaction increases when the temperature is increased.
Answer:
Original Graph
Answer with a curve for higher temperature
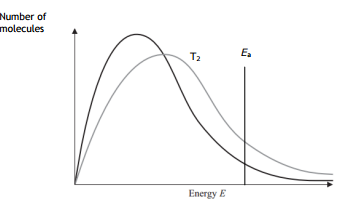
At a higher temperature more number particles have energy equal to or more than the activation energy, a more successful collision occurs. Hence the rate of reaction increases.
Use the diagram below, with further labeling as necessary, to explain why the rate of a chemical reaction increases when a catalyst is added at temperature T1.
Original Graph

Answer with new activation energy due to catalyst
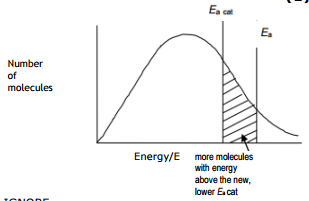
The catalyst provides an alternative path for the reaction which has lower activation energy. The new line Ea(cat) represents the new activation energy. The shaded area shows that when a catalyst is used, more particles have energy equal to or more than the activation energy. Hence more successful collisions occur, the rate of reaction increases.
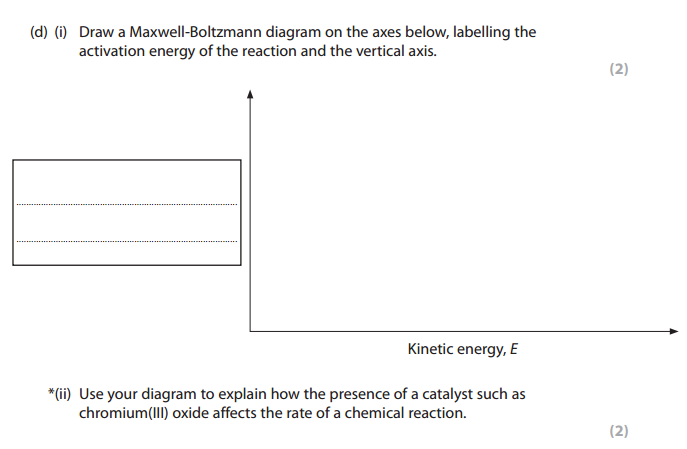
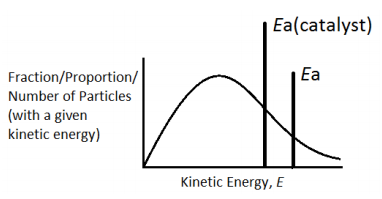
The catalyst provides an alternative path for the reaction which has lower activation energy. The new line Ea(cat) represents the new activation energy. The shaded area shows that when a catalyst is used, more particles have energy equal to or more than the activation energy. Hence more successful collisions occur, the rate of reaction increases.
At temperature T2 more particles have energy equal to or more than the activation energy, more successful collisions occur. Hence the rate of reaction increases.
Answer:
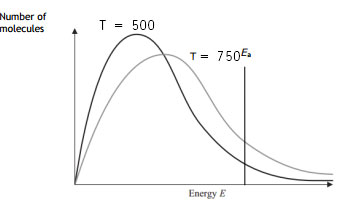
When the temperature is lowered to 500 degrees Celsius, fewer particles have energy equal to or more than the activation energy. Less number of successful collisions occurs. Hence the rate of reaction decreases.

Revise IAL Chemistry unit 2 within 1 hour & get above 90%
- No BS, to the point, quick, and easy to revise.
- Actual common questions with answers from the question paper. Get easy marks in the bag during your exam.
- Questions are divided into different topics.
- A brief discussion on each topic to get your concepts clear.
- Available in kindle ebook and paperback formats on Amazon
Shapes of molecules questions
Atoms share electrons to form a covalent bond, two or more atoms covalently bond together to form a molecule. Not all electrons of an atom are used up in covalent bonding. The remaining unused electrons form lone pairs. The pair of electrons which are used to form the covalent bond is called bond pair. In this topic we are mainly concerned about the shape of a molecule and why that molecule will have that shape. In most of the common questions, you will be asked to predict the shape name of the molecule, the angle between the bonds, and why that molecule has that specific shape.
Here are some important points which you should keep in mind:
- Lone pair- Lone pair repulsion > Bond pair – lone pair repulsion > Bond pair-bond pair repulsion
- The molecules takes up a specific shape which minimizes repulsion between the electron pairs, but maximizes the attraction.
- The shape of the molecule will depend on the number of bond pairs and lone pairs around the central atom in the molecule.
Here are some shapes, angle of molecules depending on the number of electron pairs around them.
| Number of Electrons | 2 | 3 | 4 | 5 | 6 |
| Example | Carbon dioxide | Boron chloride | Methane | Phosphorous (V) Chloride | Sulfur (VI) Fluoride |
| Bond Angle | 180° | 120° | 109.5° | 90° and 120° | 90° |
| Name of Shape | Linear | Trigonal Planar | Tetrahedral | Trigonal bipyramidal | Octahedral |
| Shape Drawing | 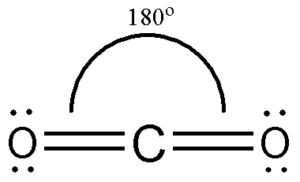 | 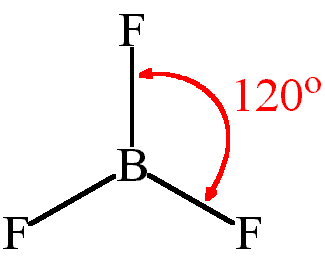 | 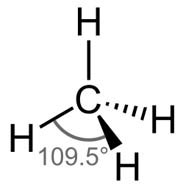 | 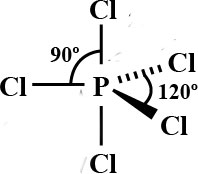 | 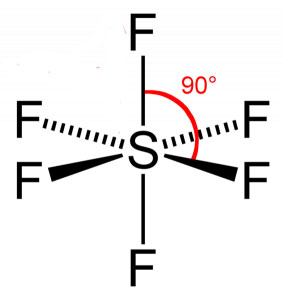 |
Related Common Questions
13 a) For BCl3, give the shape of the molecule and give the ClBCl bond angle. (2)
Answer:
Shape : trigonal planar/ triangular planar
Bond angle: 120°
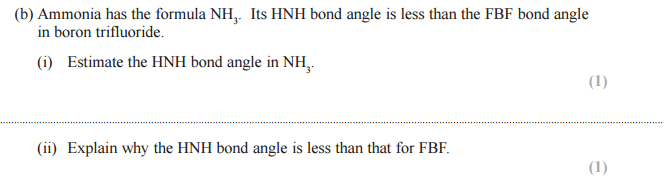
*(b) For the NCl3 molecule, draw the shape you would expect, and suggest the ClNCl bond angle. Explain why the molecule has this shape and bond angle.
Shape Bond angle :
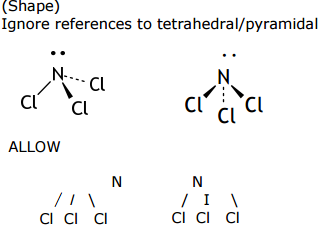
Explanation:
• Minimum repulsion (between pairs/groups of electrons /center of electron density)
• Lone pair(s) repel more than bond pair(s)
22 a (ii) Explain why ozone is a non-linear molecule
Ans: lone pair (of electrons) on the central / middle / centre oxygen atom
(I) In chlorophyll, a magnesium ion is bonded to four nitrogen atoms which are in the same plane as the magnesium. Suggest a value for the NMgN bond angle and explain how you have arrived at your suggestion. (2)
NMgN bond angle….90(°),
Explanation…..Four bonded pairs of electrons, /minimum repulsion
Reason: Three bond pairs around the carbon atom, repel each other to minimize repulsion
Shape: Trigonal planar
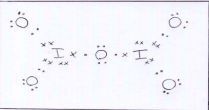

105° – 107° (1)
Pyramidal
a i) 109.5 (°)
ii)Angle: 104.5
O atom has two lone pairs (and 2 bonding pairs)
Lone pairs repel each other more than bonding pairs

Trigonal Planar
3 bond pairs of electrons
Arranged at minimum repulsion

Angle: 104.5
Justification: (Four pairs/two bonding pairs and two nonbonding pairs of electrons in) minimum repulsion/maximum separation/as far apart as possible, And lone/non-bonding pair(s) of electrons repel more (than bond pairs/CH bonds)

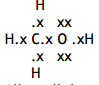
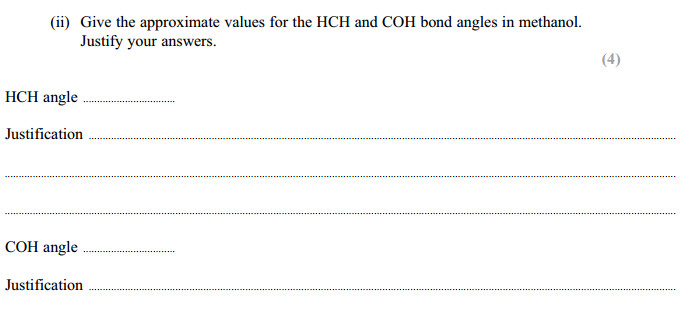
HCH angle: 109 / 109.5
Jus: Minimum repulsion / maximum separation (between four bond pairs of electrons/bonds
COH angle: 104.5 (1)
Jus: (Two) lone pairs of electrons repel more (than bonding pairs)
Some Questions so you can try yourself




Conclusion
So for now these are the common questions I have, hope you have found them helpful. I will keep on adding more common questions from time to time. I will keep on updating this blog post to make it more helpful for everyone. Please share this blog post with your friends who might need help with the A2 Chemistry Unit 2 exam. Until then have a great day, have a wonderful exam!!


Thanks for sharing valuable knowledge in chemistry
Sir, can you please make unit 4 and 5 chemistry common question ?
Thank you very much. It helps alot.
Thanks a lot sir. Do you tutor online?
Sorry no dear