What is electronegativity?
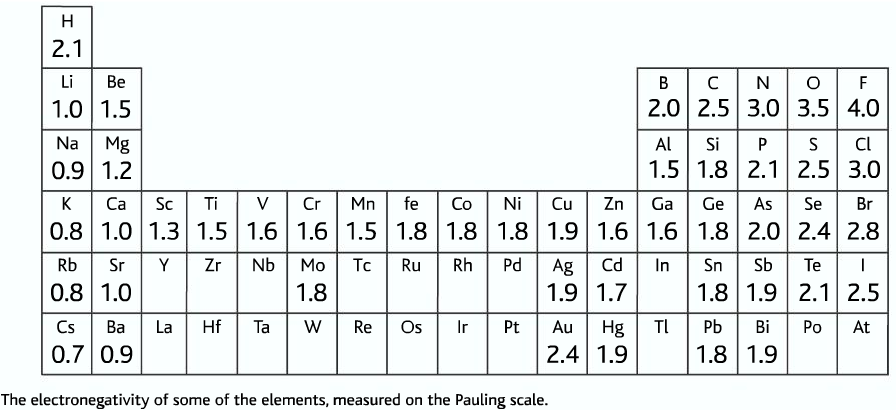
Electronegativity is the measure of the power of an atom to pull the bond pair towards itself when two atoms are involved in a covalent bond. Atoms of different elements have a different number of protons, for which different attracting powers. Electronegativity of elements increases across the period and decreases down the group. The electronegativity is measured in Pauling scale which is from one to four.
Examples:
When two atoms of the same element form a covalent bond, the bond pair is shared equally because they have the same electronegativity.

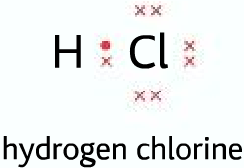
But when atoms of different elements form a covalent bond, the bond pair is shared unequally. In the
H-Cl bond the chlorine atom is more electronegative than hydrogen, pulls the bond pair towards itself.
Measurement of electronegativity:
- Electronegativity is measured on a scale called the Pauling scale.
- The Pauling scale is between 0 to 4. Zero is the lowest electronegativity while four is the highest.
Trends of electronegativity in the periodic table
- Non-metals have a higher electronegativity than metals
- Electronegativity increases across the period
- Electronegativity decreases down the group
- The most electronegative element is fluorine (4), least electronegative element is caesium (0.7)
- No electronegativity is given for noble gases because they do not form any kind of bonding
Intermediate Bonding
- Covalent bonding: The bond formed by sharing a pair of electrons. Where each electron in the pair is from different atoms.
- Ionic bonding: The metal atom(s) loses electron(s) to form cation(s). The non-metal atom(s) gains those electon(s) to form anion(s). There is high electrostatic attraction between the cation(s) and anion(s).
In real life intermediate bonding occurs, both bondings occur but to some extent.
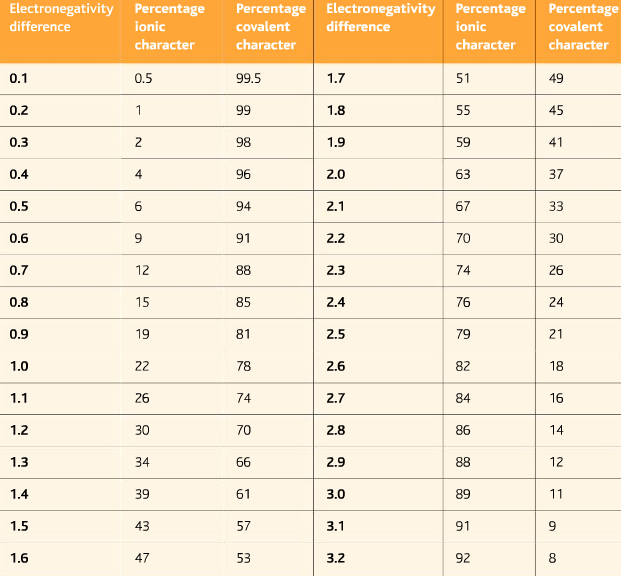
Finding the degree of ionic and covalent character
- First, find the electronegative difference between two elements.
- Then find out the electronegative difference from the table to find out the degree of covalent and ionic character.
Examples:
Calculate the percentage of ionic and covalent bonding in:
Lithium Fluoride, LiF b) Ammonia, NH3
Ans:
The electronegativities of lithium and fluorine are 1.0 and 4.0 respectively. The difference in electronegativity is 3.0
From the above table, Li-F is 89% ionic and 11% covalent
The electronegativities of nitrogen and hydrogen are 3.0 and 2.1 respectively. The difference in electronegativity is 0.9.
From the above table, the N-H bond is 19% ionic and 81% covalent
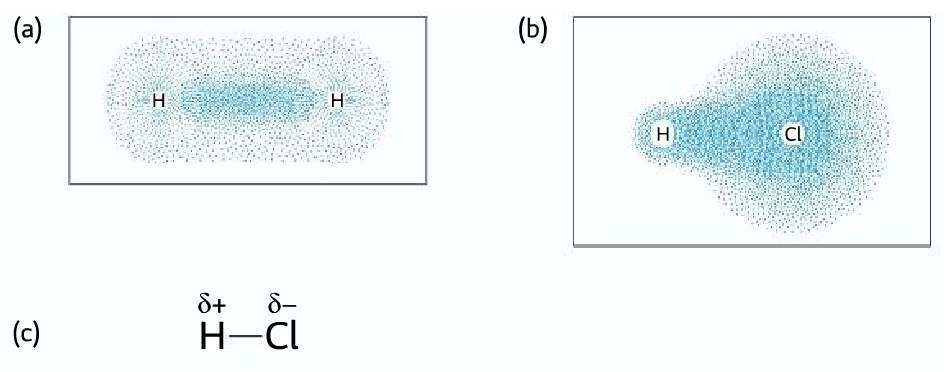
Polar Covalent Bonds
- In a normal covalent bond, the bond pair is shared equally.
- Ina polar covalent bond, the more electronegative atom pulls the bond pair towards itself, creating a partial negative charge on it. And a partial positive charge on the other atom.
To learn more about how chemical bonding works, you might find this book “Chemical Bonding (Oxford Chemistry Primers)” on Amazon very useful.

Please send me a pdf copy of this chapter..