What is everything is made of?
An atom is the most basic building block in the universe, it is the smallest you can get. Everything in this universe is made out of atoms, billions and billions of atoms. The atoms are joined together via bonds. Bonds such as an ionic bond, covalent bond, metallic bond etc. If you want to know more of how one atom attaches itself with another atom via bonding, then please read this blog post.
But the main focus of this blog would be to explain how do many atoms exist together after they have bonded together. What kind of chemical structure do they have. And also what kind of physical properties do they get after achieving that specific structure.
After fully reading this blog post, you will have crystal clear concepts of chemical structures. It will definitely help you in your chemistry exam, whether you are just a beginner or an advanced learner. This blog is for everyone!
You will learn:
- How particles exist in an ionic compound, it’s structure and what kind of physical properties do they have due to that structure.
- How particles exist in a covalent compound, it’s structure and the physical properties due to that structure.
- How particles exist in a pure metal and an alloy, it’s structure and the physical properties due to that structure.
Basically, we can divide chemical structures into two types
Giant Structure: contains a huge number of atoms or ions arranged in a particular way but the number of particles is not fixed, the ratio might be
Molecular Structure: Molecular structures only exist in covalent compounds. Some covalent compounds have molecular structure while some have giant structures. In molecular structure, two or more atoms join up to form separate molecules. Each molecule contains a fixed number of atoms. There are weak intermolecular forces between the molecules.
If you want to dive even deeper into the topic of chemical structure, then please read this amazing book “Chemical Structure and Bonding” on Amazon.
The giant structure is divided into three types:
- Giant metallic structure: this occurs only in metals. Made of cations and delocalized electrons. There is a high attraction between the cations and delocalized electrons.
- Giant Ionic structure: this occurs only in ionic compounds. This structure contains anions(-ve ions) and cations(+ve ions). There is a high attraction between the anions and cations. The number of anions and cations is not fixed, but their ratio of the number of ions is fixed.
- Giant covalent structure: In this type of structure, covalent bond continues through the whole structure.
Giant Metallic Structure: In metals, the metal atoms lose their valence electrons(outer shell electrons) to form cations and a sea of negatively charged electrons around them. The electrons which are released are delocalized which means they are free to move around. There is a high attraction between the delocalized electrons and the cations. It is this attraction which is called metallic bonding, this is what makes everything stick together like a glue.
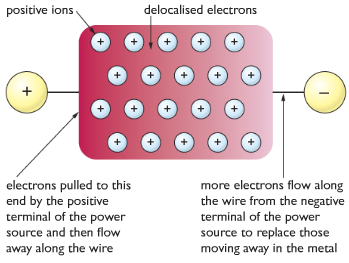
Properties of metals with reasons
| Property | Reason |
| Metals have high melting and boiling points | There is high forces of attraction between the cations and delocalized electrons, which takes lots of energy to overcome. |
| Metals conduct electricity and heat | Delocalized electrons move around to conduct heat and electricity |
| Metals are Elastic, malleable and ductile
| The cations in metal structure can slide over each other, when force is applied. |
Technical Terms Meaning
Malleable: Can be hammered into sheets. This means when you hit a metal with a hammer, it easily flattens out. The main reason for this property is that the cations can slide over each other when force is applied.
Ductile: Can be drawn into wires. This means you can apply a pulling force to the metal and draw wires from it. Also the main reason for metals having this property is that the cations can slide over each other when force is applied.
Elastic: the ability of an object to return to its original shape and size after a force deforms it. Metals show elastic properties when small forces are applied to it. Also, the main reason for metals having this property is that the cations can slider over each other when force is applied.
Metals tend to be elastic, but when a large force deforms it then it is permanently deformed.
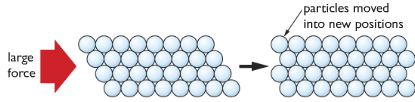
Alloys
A mixture of metals. Different metals in specific amounts (percentages) are melted and mixed together. The outcome is an alloy. Alloys are a bit different from the original metal. Please keep on reading to know more about alloys.
Property of Alloy
Alloys are harder than the original metal. Because its structure contains particles of different sizes. This reduces the ease for the particles to slide over each other. For example, iron is softer (more elastic, ductile and malleable) than its alloy steel.
Examples of some alloys and the mixtures of metals they contain
| Name of Alloy | Metals in the Alloy |
| Brass | Copper and Zinc |
| Solder | Tin and lead |
| Bronze | Copper and tin |
| Stainless Steel | Iron, chromium and nickel |
| Cupronickel | Copper and nickel |
If you are interested to learn more about the metallic structure, their ores, and their uses, then please read my blog “Extraction of Metals From Ores & Their Uses“.
Giant Ionic Structures
Positive and negative ions arranged in a regular arrangement called ionic lattice. The ionic lattice is held together by strong attractive forces between the positive and negative ions.
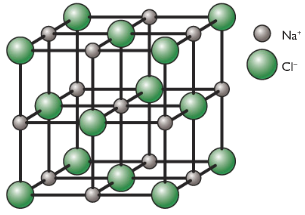
Example: Lattice of NaCl
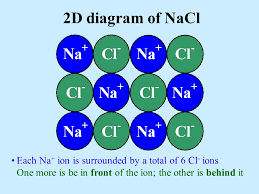
3D Lattice of NaCl
Each Na+ ion is surrounded by 6 Cl– ions:
Properties of ionic compounds with reasons
| Property | Reason |
| Ionic compounds have high melting and boiling points | There is high forces of attraction between oppositely charged ions. |
| Most ionic compounds are crystalline | This is because the ions in the ionic compound are arranged in a regular structure. Which is also called ionic lattice. |
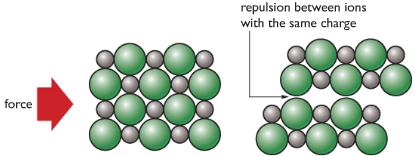
| Ionic Substances are usually brittle. Meaning that it easily breaks apart when force is applied. | Force causes like charges to face each other, repel and break apart. |
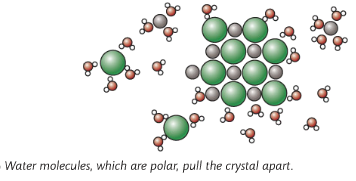
Most ionic compounds are soluble in water, but insoluble in organic substances
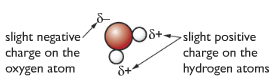
Water molecules are polar (slight positive charge at one end of the molecule and slight negative charge at the other end of the molecule). When an ionic substance is put in water, the negative part of the water molecule pulls on the positive ion, and the positive part of the water molecule pulls the negative ion, thus breaking up the ionic substance causing them to dissolve. But some compounds are insoluble in organic substances as they are non-polar
Ionic substances conduct electricity in solution or molten state but not in the solid state.
Reason: Ions are free to move around in solution or molten state to conduct electricity, but not in
Giant Covalent Structures
It is a macromolecular structure, a large number of covalent bonds in a single structure. Let’s have a look at the example of diamond and graphite to have a better understanding.
Diamond
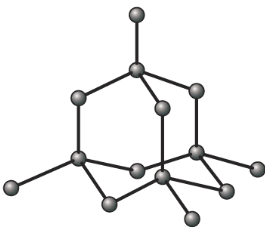
Properties of diamond with reasons
| Property | Reason |
| Very hard, with high melting and boiling point | Strong carbon-carbon covalent bonds continue throughout the whole structure. |
| Diamond doesn’t conducts electricity | No delocalized electrons, all electrons are used up in each carbon |
| Diamond is insoluble in water | Strong covalent bonds between carbon atoms |
Uses of diamond: jewelries, cutting glass.
Graphite
In graphite, e
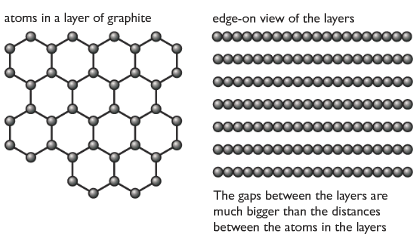
Properties of graphite with reason
| Property | Reason |
| Soft and slippery | Layers can slide over each other |
| High melting and boiling points | Strong carbon-carbon covalent bonds in each layer |
| Conducts electricity | One electron in each carbon atom is free, delocalized, can move around in layers to conduct electricity. |
Graphite is less dense than diamond, due to more spaces between layers.
Simple molecular structure
Separate molecules, containing a fixed number of atoms, with weak intermolecular forces between the molecules. Strong covalent bonds between atoms in a molecule, but weak intermolecular forces among molecules. (Eg. Cl2, CO2, N2 )
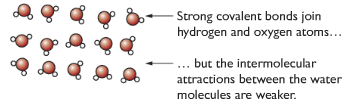
Properties of molecular substance with reason
| Property | Reason |
| Low melting and boiling points | Weak intermolecular forces between molecules |
| Doesn’t conduct electricity | Molecules don’t have overall charge |
| Tends to be insoluble in water and soluble in organic substances | Due to similar forces between molecules |
Elements: pure substances, which contains atoms all having the same proton number. For example, a pure metal sodium, oxygen, and even diamond contain only one type of atom.
Compound: Two or more elements in fixed proportions chemically bonded together. Eg. Water, carbon dioxide, and sodium chloride.
Mixture: Two or more substances mixed physically. Eg: sand and salt, salt and water.
Differences Between Mixtures and Compounds
| Mixture | Compound |
| Proportion not fixed (eg: a mixture of salt and water) | Fixed proportion (eg: H20, Cl2) |
| Properties depend on the substances in mixture. | New unique property |
| Can be easily separated | Hard to separate, needs complex methods |
| No energy changes occur | Usually there is an energy change |
Conclusion
Thank you for reading this blog. I hope now your basics about chemical structures is crystal clear. Now it would be easier for you to digest the more complicated topics which are based on the concepts of chemical structure.
If you find this blog helpful, then please feel free to share it with your friends who might need help.

Pingback: Preparation of salts and solubility of salts - IGCSE And IAL Chemistry