Whenever you hear the word “salt”, what comes to your mind? Most probably the table salt. The white powder which we add to our food to make them taste better. After all who doesn’t like perfectly salted fish n chips! But in chemistry, salts have a much deeper meaning.
In this blog, I am going to discuss in-depth “salts”. After completely reading this blog, you will have a crystal clear idea about the following concepts:
- What is a salt
- Soluble and insoluble salts
- How to identify whether a type of salt is soluble or insoluble.
- How to prepare different types of soluble salts.
- How to prepare different types of insoluble salts
- Different types of experiments, tests and what not!
Just properly go through this blog, get your concepts clear and show the nerd in your class that it is you who is the real boss!
What are salts?
In chemistry, salt is just a nickname for ionic compounds. If you are flabbergasted by the word ionic compound and have no clue about it at all, in that case, you better have a look at this article about ionic bonding.
Most ionic compounds or salts are soluble in water because water contains polar molecules. In simple terms, a polar molecule is a molecule with one end slightly positively charged and the other end slightly negatively charged.
So when you place an ionic compound in water, the positive end of the water molecules pulls on the negative ion while the negative part of the water molecule pulls the positive ion of the ionic compound. In this way, the water molecules break apart the ionic lattice and dissolve it. Obviously, there is more to it than that, but for now, just assume this simple story is what happens. You can also go through this article where I explain in detail with diagrams that how water dissolves an ionic compound.
Before you dive into this information goldmine about salts, I think you better have a quick look at this article about acid, there are some important reactions I have mentioned there. This will make sure that you make full use of this article.
How to Determine whether a salt is soluble or insoluble in water?
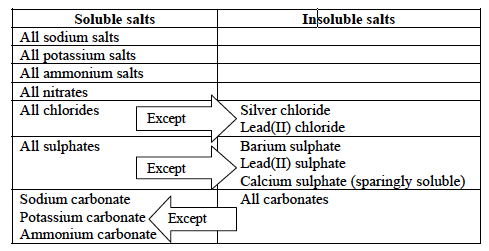
To determine whether salt is soluble in water or not, we need to keep in mind the following facts:
**PLEASE KEEP IN MIND THAT THESE FACTS ARE HIGHLY IMPORTANT. JUST BY KNOWING THESE FACTS, YOU CAN EASILY STATE WHETHER A SALT IS SOLUBLE OR INSOLUBLE**
- All sodium, potassium, and ammonium salts are soluble in water. Example: Na2CO3, K2SO4, NH4Cl salts are all soluble in water.
- All nitrate salts are soluble in water. Example: Mg (NO3)2, Cu (NO3)2 and Fe (NO3)3 salts are all soluble in water.
- Most common chloride salts are soluble in water except silver chloride (AgCl), lead (II) chloride (PbCl2).
- Most common sulfate salts are soluble in water except, lead (II) sulfate (PbSO4), barium sulfate (BaSO4) and calcium sulfate (CaSO4).
- Only sodium, potassium and ammonium carbonates (Na2CO3, K2CO3 and (NH4)2CO3) are soluble in water, rest is insoluble.
- Most metal hydroxides are insoluble in water except the sodium, potassium and lithium metal hydroxides (NaOH, KOH, and NH4OH).
Examples: How I determine which salts are soluble and which are insoluble.
| Salt Name | Solubility |
| NaCl | soluble( all sodium salts are soluble) |
| PbSO4 | insoluble (lead(II)Sulfate is insoluble as we learned) |
| CaCO3 | insoluble( we learned that all carbonate salts are insoluble except (Na2CO3, K2CO3, and (NH4)2CO3, so this salt is insoluble) |
| K2SO4 | soluble (all potassium salts are soluble) |
| AgCl | insoluble (we learned that silver chloride is among one of the insoluble chloride salts) |
NOW YOU TRY! State whether the following salts below are soluble or insoluble
- CuCO3
- BaSO4
- NH4Cl
- Mg(NO3)2
- K2SO4
- PbCl2
Making Salts
In order to make life easier, chemists have divided salts into three types:
- Soluble Salts
- Insoluble Salts
- Potassium, Sodium and Ammonium salts
Dividing salts into three types makes it easier to conceptualize and prepare them.
Soluble salts – To make soluble salts, simply react an acid with access metal (make sure it is a metal of moderate reactivity, should lie between magnesium and iron in the reactivity series
Insoluble salts– Make insoluble salts by the precipitation method (mix two solutions, each solution contains respective ion, when the ions meet when solutions are mixed, they form the insoluble salt)
Potassium, sodium, and ammonium salts – by titration method( by the neutralization reaction between an acid and an alkali)
To better understand the relationship between Acid, Base and Salt I would recommend you read this book “Chemistry: Acids, Bases and Salts (2nd Ed.)” from Amazon.
Here is the summary of which steps to follow while making a salt.
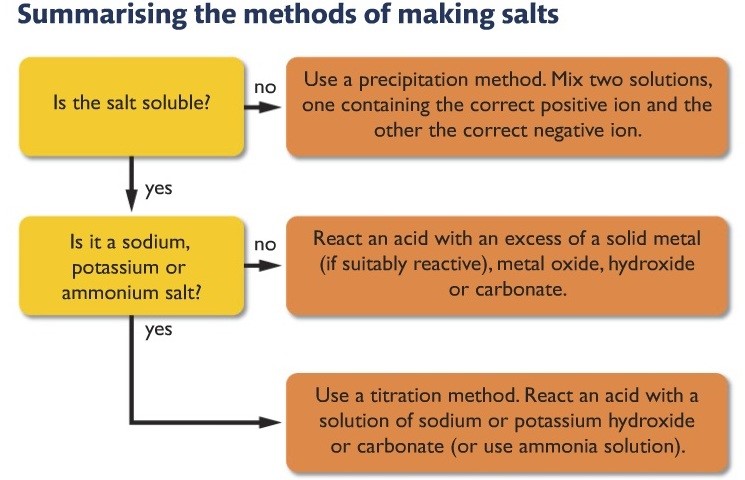
Reactions for makings soluble salts (except sodium, potassium and ammonium salts)
Acid+ metal→ salt+ hydrogen gas
Please make sure to use metals between magnesium and iron in the reactivity series, because it could be dangerous to use a more reactive metal.
Example 1: Mg +2 HCl → MgCl2 + H2
Acid+ metal hydroxide/metal oxide → salt + water
Example 2: CuO + H2SO4 → CuSO4 + H2O
Acid + metal carbonate→ salt+ water+ carbon dioxide gas
Example 2: 2HCl + CaCO3 → CaCl2 + H2O + CO2
Making magnesium sulfate crystals
From the things we have learned so far, we can easily state that magnesium sulfate is a soluble salt. Magnesium is a moderately reactive metal, so we can directly react to magnesium with an acid to make the salt.
To get the sulfate(SO42-) ions we use sulfuric acid( H2SO4).
We react excess magnesium with dilute sulfuric acid to make sure that all the acid is used up and no H+ ions are left behind.
The following reaction below occurs between magnesium and sulfuric acid:
Mg(s) + H2SO4 (aq) → MgSO4 (aq) + H2 (g)
During the reaction, we see the bubbling of an invisible gas. That invisible gas is hydrogen. Excess magnesium is left behind in the solution of magnesium sulfate. The magnesium simply sits in the bottom of the beaker as a deposit.
To obtain crystals of magnesium sulfate we need to follow the crystallization procedure because magnesium sulfate needs water of crystallization to form crystals. If we heat the solution of magnesium sulfate strongly, only powder of anhydrous magnesium sulfate will be left behind.
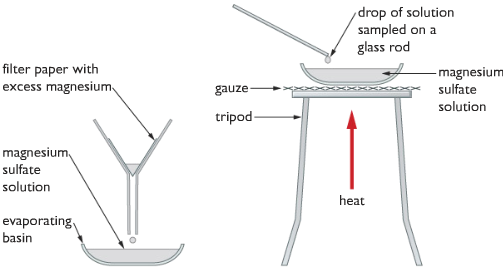
Crystallization Procedure
- Firstly, filter off the excess magnesium from the magnesium sulfate solution.
- Take some magnesium sulfate solution into an evaporating dish.
- Heat the magnesium sulfate solutions in the evaporating dish gently, meanwhile dipping a glass rod time to time while heating. When the solution will be concentrated enough, crystals of magnesium sulfate would start forming on the tip of the glass rod.
- Then we stop heating and then leave the concentrated solution to lose more water by evaporation. Then crystals of MgSO4 start to form by itself.
MgSO4 (aq) + 7H2O (l) → MgSO4.7H2O (s)
Making Copper (II) Sulfate crystals
We cannot react copper with acid because copper is less reactive than hydrogen. Copper does not react with any acid at all! Maybe a little bit of highly concentrated sulfuric acid, but that’s dangerous! However, we can
CuO(s) + H2SO4(aq) → CuSO4(aq) +H2O(l)
We use sulfuric acid because we need the sulfate(SO42-) ion from the sulfuric acid.
In a beaker of dilute sulfuric acid, add excess black powder of copper (II) oxide. After the reaction occurs, a blue solution of copper (II) sulfate is formed. Excess black copper (II) oxide powder is left behind as a deposit at the bottom of the beaker.
Firstly filter, to remove the excess copper (II) oxide powder, and yet again follow the crystallization procedure as I described before.
CuO(s) + H2SO4(aq) → CuSO4(aq) +H2O(l)
CuSO4 (aq) + H2O (l) → CuSO4.5H2O(s)
Making sodium, potassium and ammonium salts
When making sodium, potassium, or ammonium salts, we face an odd problem, which is that all of their compounds/salts are soluble in water.
For example, if you
So to solve this problem, we need to do a titration, to make salts of these kinds. Meaning we need to react exactly the required amount of acid with an alkali. So no leftover reactants will remain behind to contaminate our salt solution.
Making Sodium Sulfate crystals
To make sodium sulfate crystals, we can react sodium hydroxide (NaOH) with sulfuric acid (H2SO4)
NaOH(aq) + H2SO4(aq) → Na2SO4(aq) + 2H2O(l)
Firstly use a pipette to transfer 25 cm3 of sodium hydroxide to a conical flask.
Then add a few drops of methyl orange indicator into the conical flask.
The colors of methyl orange are given below
| Alkaline | yellow |
| Neutral | Orange |
| Acidic | Red |
When the few drops are added to the conical flask it turns yellow, as it contains an alkaline solution of sodium hydroxide.
Use a burette to add dilute sulfuric acid to the sodium hydroxide solution. When the sulfuric acid will neutralize all of the sodium hydroxides, the color will change from yellow to orange. But if too much acid is added the solution will red, and we will have to repeat the whole thing again.

When the indicator color turns orange, note down the volume of acid used.
In a separate beaker mix 25 cm3 of NaOH with the volume of acid used. Because the titration solution is now contaminated with the indicator methyl orange.
The sodium sulfate salt also requires water of crystallization to form crystals, so we must follow the crystallization procedure yet again.
Na2SO4 (aq) + 10H2O (l) → Na2SO4.10H2O(s)
Making Sodium Chloride crystals
To make sodium chloride crystals, you can react sodium hydroxide with hydrochloric acid, by titration method.
NaOH (aq) + HCl(aq) → NaCl(aq) + H2O(l)
Sodium Chloride does not require water of crystallization to form crystals, so just heat the solution to evaporate all the water, sodium chloride crystals will remain behind.
Making ammonium sulfate [(NH4)2SO4] crystals
To make (NH4)2SO4 crystals react aqueous ammonia (NH4OH) with sulfuric acid (H2SO4):
NH4OH (aq) +H2SO4 (aq) → (NH4)2SO4 (aq) + H2O (l)
OR NH3 (aq) + H2SO4 (aq) → (NH4)2SO4 (aq)
Ammonium salts do not have water of crystallization, but still, we must follow the
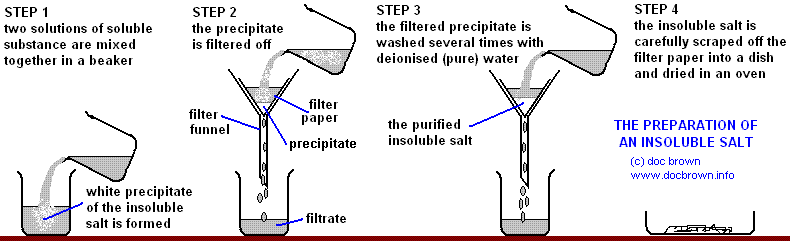
Making Insoluble Salts
To make insoluble salts, we will follow the precipitation method.
The precipitate is a fine solid that is formed by a chemical reaction involving liquids or gases.
Suppose you want to make a salt AB, where A+ is the cation and B– is the anion. So we take a solution that contains A+ ions and takes another solution that contains B– ions, then we mix the solutions in a beaker, hence we get the precipitate of the salt AB.
But to get an A+ ion in a solution, we must dissolve a soluble salt in the water which would release the A+ ions, the same thing goes for B+.
Making Silver Chloride (AgCl)
From our previous lessons now we know that silver chloride is an insoluble salt, so we must follow the precipitation method to make it.
We must take a solution that will contain Ag+ ions, so we can dissolve silver nitrate (AgNO3) to obtain it. Because all nitrate salts are soluble in water, so they would dissolve to release ions of Ag+
We also must take a solution which will contain Cl– ion, so we can dissolve sodium chloride (NaCl) to obtain it, because all sodium salts are soluble in water, so it would dissolve to release ions Cl–
Overall equation: AgNO3(aq) + NaCl(aq) → AgCl(s) + NaNO3(aq)
Ionic equation: Ag+(aq) + Cl–(aq) → AgCl(s)
Note: The ionic equation is known as a precipitation reaction.
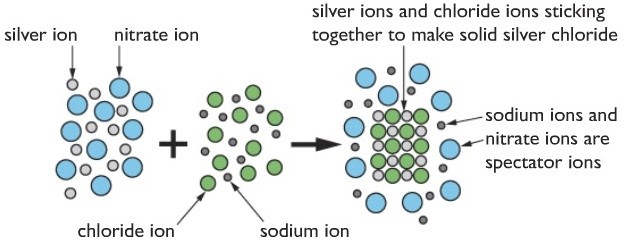
Explanation of how this occurs
Silver nitrate contains silver ions and nitrate ions in the solution. The positive and negative ions are attracted to each other but the attractions aren’t strong enough to make them stick together. Similarly, the sodium chloride solution contains sodium ions and chloride ions- again, the attractions aren’t strong enough for them to stick together in the water.
When you mix the two solutions, the various ions meet each other. When silver ions meet chloride ions, the attractions are so strong that the ions clump together and form a solid. The sodium and nitrate ions remain in the solution because they aren’t sufficiently attracted to each other.
Making pure dry Barium Sulfate
Take a solution containing Ba2+ ions, for example, Barium nitrate [Ba(NO3)2]
Take solution containing SO42- ions, for example, sodium sulfate [Na2SO4]
Mix the solutions in a beaker and the precipitate of BaSO4 will be formed.
Overall equation: Ba(NO3)2 (aq) + Na2SO4 (aq) → BaSO4(s) +2 NaNO3(aq)
Ionic equation: Ba2+(aq) + SO42-(aq) → BaSO4(s)
From the overall equation, we can see that the precipitate of BaSO4 is in the solution of sodium nitrate,
Firstly we must filter to remove the BaSO4, and then wash it with distilled water to remove any solution
clinging on to it. Then just leave it to dry.
Making Pure dry lead(II)Iodide[insoluble salt]
Take a solution containing Pb2+ ions, for example lead(II)nitrate [Pb(NO3)2]
Take a solution containing I– ions, for example, sodium iodide [NaI]
Mix the solutions in a beaker and the precipitate of PbI2 will be formed.
Overall equation: Pb(NO3)2(aq) +2 NaI(aq) → PbI2(s) + 2NaNO3(aq)
Ionic equation: Pb2+(aq) + 2I–(aq) → PbI2(s)
Follow the same procedure to obtain the pure dry sample of lead(II)Iodide.
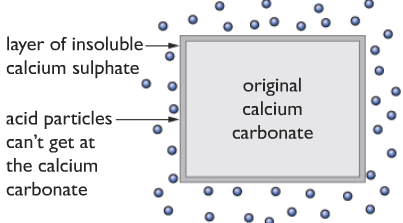
Important: Calcium carbonate should react with both the hydrochloric acid and the sulfuric acid. But the reaction stops in the middle! Why? Read below to learn!
CaCO3(s) + HCl(aq) → CaCl2(aq) + CO2(g) + H2O(l)
CaCO3(s) + H2SO4(aq) → CaSO4(s) + CO2(g) + H2O(l)
In reality, the calcium carbonate reacts normally with hydrochloric acid, fizzing of carbon dioxide gas occurs. But in the case of sulfuric acid if we use big lumps of calcium carbonate, nothing seems to happen, we may see a few bubbles of carbon dioxide only, but why?
Answer: If we recall, we can see that calcium sulfate is insoluble in water. When the calcium carbonate reacts with the sulfuric acid, the insoluble calcium sulfate forms a layer of insoluble calcium sulfate around the calcium carbonate, which prevents the acid from getting to the calcium carbonate and reacting with it.
Conclusion
Thank you for reading this informational blog. We have covered a ton of important concepts about salts. These will definitely help you in your exams, doesn’t matter what level of chemistry you are studying.
If you have any queries, please feel free to comment below with your questions.
If you found this blog useful please feel free to bookmark it in your browser for later use. If you have a friend who desperately needs help regarding this topic, send them the link to this blog post.
Before you leave, have a look at the other chemistry blogs I have written. You may find something useful!


Superb blog you have here but I was wondering if you knew of any discussion boards that cover the same topics talked about here? I’d really like to be a part of group where I can get feedback from other experienced individuals that share the same interest. If you have any suggestions, please let me know. Thank you!
Pingback: Cialis 20mg prix en pharmacie
Pingback: writeessay
It is an informing blog and summorized in the right manner in my opinion. The only thing that i thought you should have imputed was the prepration of soluble acidic salts. I know it is the same method of titration but what makes the results different? Meaning how are all the H+ ions are replaced compared to an acidic salt where some of the H+ ions are replaced.