What is in the air?
The air which we inhale and exhale every day is not a pure gas, it is a mixture of several types of gases, some are elements and some are compounds. In the unpolluted air, the four major gases present in air the are oxygen, nitrogen, argon, and carbon-dioxide, oh also water vapor is present too.
The fraction of each gas in the air is shown in the table below
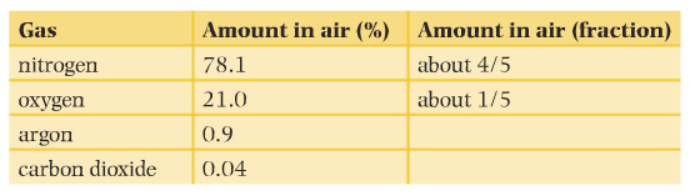
From the following table, we can see that air contains about 21% oxygen. Oxygen is the active component of air, the only thing which reacts, the other gases present are not active and do not react, or already have reacted.
But how can we prove that air contains about 21% oxygen?! Don’t worry, there are two fun experiments to prove that.
Experiment 1: Reaction of Oxygen with Copper
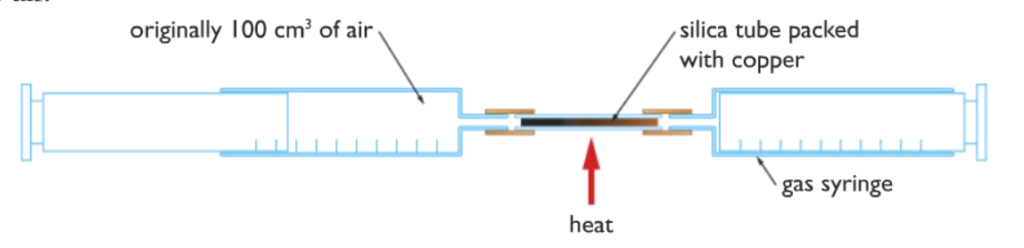
Firstly, set up the following apparatuses as shown above. One of the gas syringes contains 100 cm3 of air and the other syringe is empty. Use the gas syringes to pass the 100 cm3 air above the copper while heating it from below. The copper turns black as copper(II)oxide forms on top of it. Stop the procedure when the copper does not turn darker anymore, this indicates that all the oxygen in the trapped air is used up, no more oxygen left to react to form dark copper(II)oxide.
After you have stopped the procedure, let the air inside cool down because when heated the air expands and the volume is increased, so with the hot air we will not get the accurate volume.
After the air has cooled down, we will see that its volume has decreased by almost 21 cm3 out of the 100 cm3 this indicates that the air is composed of 21% oxygen gas.
Cu(s) + O2 ⟶ 2CuO(s)
Experiment2: Using the rusting of Iron
When iron rusts, it reacts with both the air and water. Simply to say, when iron rusts it used up the oxygen gas in the air.
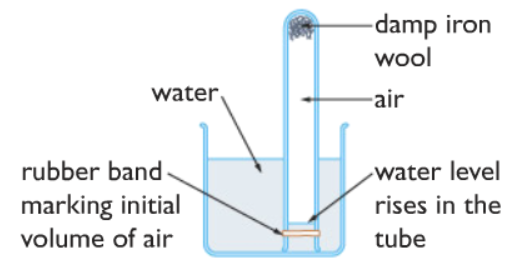
The apparatus is set up as shown in the diagram above. The initial water level is marked by the rubber band. After the damp iron wool is left like this for a week the oxygen in the air is used up as iron reacts with both oxygen and water to form rust. The volume of the air decreases due to the use up of oxygen gas, this causes the water level to rise up inside the tube. The new water level is marked with another rubber band. The initial volume of air can be found out by filling up the test tube with water up to the rubber band mark for the initial volume, then measuring the volume of the water using a measuring cylinder, the volume of the water is the volume of air. In the same way, the final volume can be measured.
Suppose, the initial volume of air is 15 cm3 and the final volume is 12 cm3 . Then the volume of oxygen gas is 15- 12 = 3 cm3
Hence the percentage of the volume of oxygen in the air is = (15-12)/15 x 100% = 20 % ( This is almost 21%)
How to make oxygen gas in the laboratory
Oxygen gas is made in the lab by the catalytic decomposition of hydrogen peroxide, manganese(IV) oxide is used as the catalyst.
2H2O2 (aq) ⟶ 2H2O (l) + O2(g)
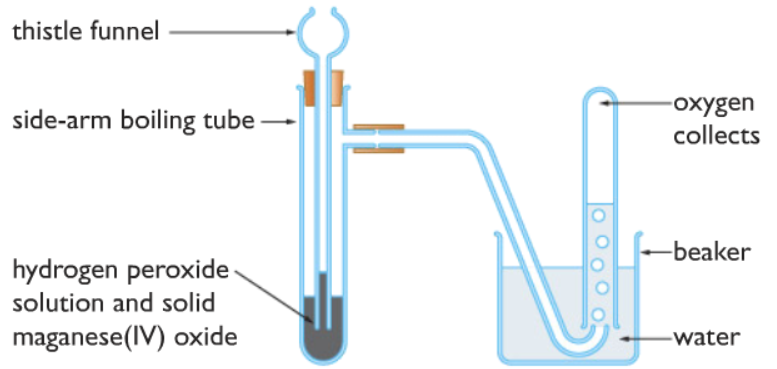
Test for oxygen gas: oxygen relights a glowing splint (no pop sound!)
Burning elements in oxygen or air
Elements burn in both oxygen and air, but they burn more intensely in pure oxygen.
Burning Magnesium: Magnesium burns in air with a bright white flame, the flame is even brighter when burned in pure oxygen.
2Mg(s) + O2(g) ⟶ 2MgO(s)
Burning Sulfur: Sulfur burns in air with an almost invisible blue flame to form colorless, poisonous sulfur dioxide gas. The sulfur burn with even brighter blue flame in pure oxygen.
S(s) + O2(g) ⟶ SO2(g)
Burning Carbon: Carbon burns in air when strongly heated, it burns with small orange-yellow flames along with some sparks.
C(s) + O2(g) ⟶ CO2(g)
What are oxides?
Oxides are compounds of oxygen, when elements react with oxygen they form oxides. For example, when carbon reacts with oxygen, carbon dioxide is formed, it is an oxide.
There are two types of oxides, metallic oxides, and non-metallic oxides. When oxygen reacts with non-metals, non-metallic oxides are formed. When oxygen reacts with metals, metallic oxides are formed. If you are confused about metals and non-metals, please review the periodic table article.
To learn in more detail about the chemical properties and uses of metal oxides, you can read this book “Metal Oxides: Chemistry and Applications (Chemical Industries Book 108)” on Amazon.
Metallic Oxides
Most metallic oxides do not dissolve in water, those which does forms an alkaline solution. For example, Magnesium Oxide dissolves in water to form an alkaline solution.
MgO(s) + H2O(l) ⟶ Mg(OH)2 (s and aq)
Non-metallic Oxides
Most non-metallic oxides dissolve in water, when they do they form an acidic solution. For example, sulfur dioxide dissolves in water to form sulfurous acid.
SO2(g) + H2O(l) ⟶ H2SO3(aq)
Simply put, metallic oxides are basic while non-metallic oxides are acidic. If you are confused about acids and bases, then please review the article I have written on acids.
What is acid rain?
Acid rain is formed when non-metallic oxides in the air react with rain. Rain is already slightly acidic due to carbon dioxide dissolving in it, but it becomes even more acidic when pollutant gases such as sulfur dioxide and nitrogen dioxide dissolve in it. Sulfur dioxide is produced mainly in factories and power stations where coals are burned, coal contains some sulfur, when coal is burned to produce energy sulfur also burns with it to produce sulfur dioxide. On the other hand, nitrogen dioxide is mainly produced in car engines, wherein the high-temperature environment, the nitrogen is forced to react with oxygen.
What are the problems with acid rain?
Acid rain causes plants to die, monuments made out of limestones die and also fish die due to the change in pH in water caused by acid rain.
Solution: Non-metallic oxides such as sulfur dioxide and nitrogen dioxides can be removed in factories by scrubbing. In car engines, the catalytic converter is used which turns oxides of nitrogen into harmless nitrogen gas.
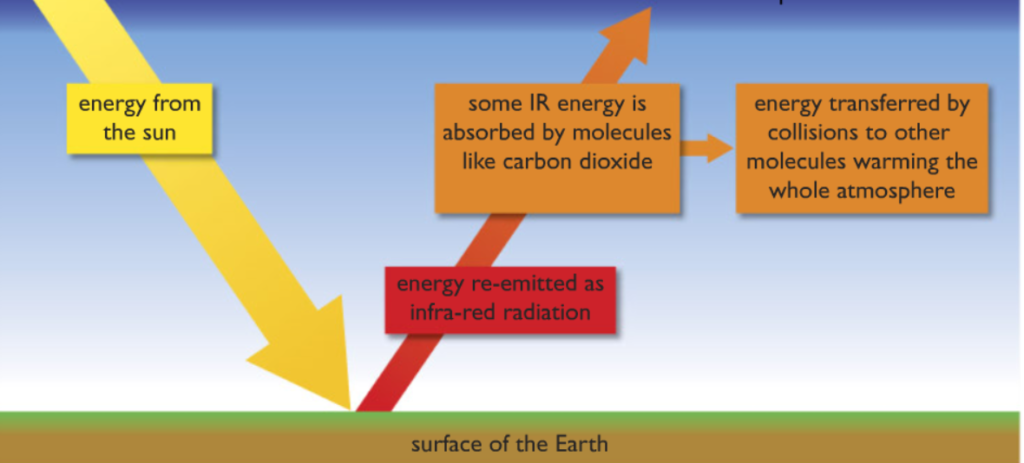
How Carbon dioxide gas causes Global Warming
When the Infrared from the sun passes through the atmosphere of the earth and is absorbed by the earth. The earth then re-radiates the Infrared but with a longer wavelength. The long wavelength infrared is trapped by greenhouse gases such as carbon dioxide This heats up the atmosphere. Though it is a natural process, it is accelerated due to the excessive burning of fossil fuels. To learn more about global warming please view my article on Green Chemistry.
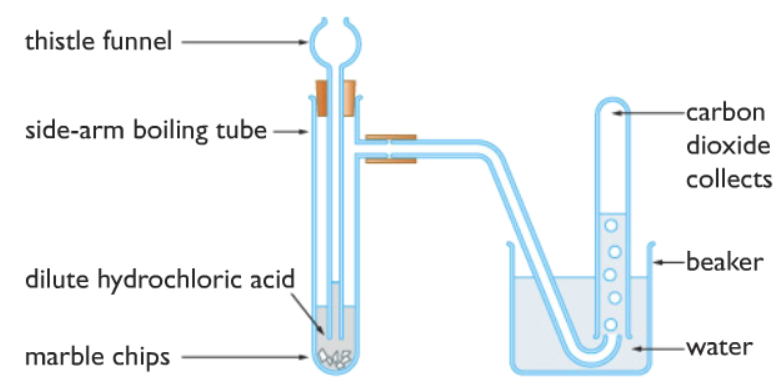
How to make carbon dioxide gas in the laboratory
Carbon dioxide is usually made in the laboratory by reacting calcium carbonate with dilute hydrochloric acid.
CaCO3(s) + 2HCl(aq) ⟶ CaCl2(aq) + H2O(l) + CO2(g)
Test for Carbon dioxide gas: Carbon dioxide turns lime water milky.
Properties of carbon dioxide: Carbon dioxide gas is colorless, odorless, slightly soluble in water and heavier than air.
Uses of Carbon dioxide: Carbon dioxide is used in fizzy drinks and fire extinguishers


Chemistry articles are interesting and informative. Thank you.