In this IAL Chemistry blog, we are going to go through the basic to advanced concepts about REDOX. Concepts such as: what is REDOX, Oxidation, Reduction, Oxidation Number, how to balance Half equations to form a complete ionic reaction, etc. So let’s dive into this fun blog and learn more about REDOX reactions!
What is REDOX?
Redox is a term used in chemistry to describe certain types of reactions. Redox reactions are reactions in which both Oxidation and Reduction takes place. The part “RED” represents reduction, while the part “OX” represents oxidation.
I will explain in full details the terms oxidation and reduction, but first, we would need to understand the concept of oxidation number.
The oxidation number is a term used to simply keep track of the number of electrons, that how many electrons are lost or gained during a chemical reaction.
Reduction VS Oxidation
To get a better understanding of the rest of the contents, let’s keep things simple and keeping the definitions of oxidation and reduction in terms of gain or loss of electrons.
Oxidation: A substance is said to undergo Oxidation or is oxidized when it loses electrons or an electron.
Reduction: A substance is said to undergo Reduction or is reduced when it gains electrons or an electron.
Finally as mentioned before, a reaction in which both oxidation and reduction occur, that reaction is known as REDOX reaction.
If you want to dive in and learn about REDOX reactions in great detail, then I would recommend you check out this book “A Better Way to Learn Redox Chemistry” on Amazon.
Example of a REDOX Reaction
Let’s analyze the reaction between Sodium and Chlorine to form Sodium Chloride, it is an ionic compound (if you need to learn more about ionic bonding and how it is formed, then please view this article “Chemical Bonding“.)
When sodium and chlorine react, sodium loses one electron while chlorine gains one electron. Sodium is oxidized as it loses an electron and chlorine is reduced as it gains an electron.
Both Oxidation and reduction occur, hence this reaction is known as a redox reaction.
Na(s) + Cl2 (g) → NaCl(s)
Na → Na+ + e–
Cl + e– → Cl–
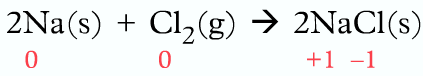
Understanding the term Oxidation number
As discussed, the oxidation number keeps track of the number of electrons gained or lost. When a species loses an electron, its oxidation number increases, on the other hand when a species gains an electron, its oxidation number decreases.
Let’s use the reaction between sodium and chlorine to understand the term oxidation number.
Sodium atom loses an electron, it’s oxidation number increases from 0 to +1.
Chlorine gains an electron, it’s oxidation number decreases from 0 to -1.
Some Rules about Oxidation Number:
- The Oxidation number of any uncombined element is zero: the oxidation number of an element is zero because it has neither gained or lost an electron, the oxidation number simply represents the change in the number of electron or electrons.
- The oxidation number of an uncombined ion is the same as its charge: for example, an uncombined ion such as Na+ will have an oxidation number of +1. Another example, an uncombined ion of O2-(Oxide ion) will have an oxidation number of -2.
- The sum of all the oxidation numbers in a molecule is zero: For example, in a carbon dioxide molecule, the sum of oxidation numbers of carbon and the oxygen would be zero. In the case of a complicated ion such as NO3–(nitrate ion), the sum of the oxidation number would be equal to the charge of the complicated ion, in this case -1. Oxidation numbers in covalent compounds would be discussed in details in the latter part of this article.
- Fluorine always has an oxidation number of -1: This statement means that whenever fluorine undergoes a reaction, it always ends up with an oxidation number of -1. This concept would be cleared up when we will see some examples of how to calculate the oxidation number.
- Hydrogen always has an oxidation number of +1, except in metal hydrides (in those cases the oxidation number is -1)
- When oxygen forms a compound it always ends up with an oxidation number of -2: some exceptions are when oxygen is in peroxides such as H2O2(Hydrogen Peroxide, in this case, the oxidation number of oxygen is -1), also when oxygen combines with fluorine it ends up with a positive oxidation number!
- Chlorine always ends up with an oxidation number of -1 when it is a compound: one exception is when chlorine forms a compound with fluorine. In that case, chlorine ends with a positive oxidation number. This is because fluorine is more electronegative than chlorine. To learn more about electronegativity, please view this article “Electronegativity and Bond Polarity“
- Group 1 elements always ends up with an oxidation number of +1 when they form compounds, group 2 ends up with +2 and group 3 ends up with +3: for example, Na in group 1 becomes Na+ which has an oxidation number of +1, Ca in group 3 becomes Ca+2 which has an oxidation number of +2 and Al in group 3 becomes Al+3 which has an oxidation number of +3.
The balance of Oxidation numbers:
When a reaction occurs, electrons are being gained and lost. The number of electrons which are being gained by a species will be equal to the number of electrons lost by another species. There won’t be more and less electron involved. Hence the sum of the oxidation number should be equal on both sides.
Example 1:
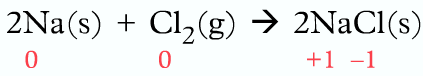
On the left-hand side Na and Cl2, both have an oxidation number of 0 because they are uncombined elements, according to the rules of oxidation number. The sum of oxidation number on the left hand side is = 0 + 0 = 0
On the right-hand side there are two sodium ions and two chloride ions, 1:1 ratio, so we can just assume that there are one sodium and one chloride ion, hence the sum of oxidation number on the right-hand side is = +1-1 = 0
So both the sum of oxidation numbers equals on both sides of the reaction as expected.
Example 2:
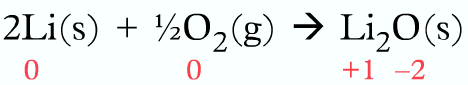
On the left-hand side, the oxidation number of lithium and oxygen are zero, because they are uncombined elements. So the sum of the oxidation number in the left-hand side is 0+0 = 0
On the right-hand side, Li becomes Li+ and 1/2O2 becomes O2-, two lithium-ions with an oxidation and oxidation number of +1 each and one oxide ion with an oxidation number of -2. Sum of oxidation number in the right-hand side = (2 x +1) + (-2) = 0. So the sum of oxidation numbers is equal on both sides as expected.
What are Half-equations?
Half-equations break down a normal reaction into two parts and shows which species have gained electron(s) and which species have lost electron(s). In this way, it is easier to determine which species is reduced or oxidized.
Let’s take the example of the reaction between sodium and chlorine yet again:
Normal Equation: 2Na + Cl2 → 2NaCl
Here the Na becomes Na+
And, Cl becomes Cl–
So the Half-equations are:
Na → Na+ + e–
Cl2 + 2e– → 2Cl–
From these half-equations, it can be seen that sodium is oxidized as it loses an electron, and chlorine is reduced as it gains two electrons. But you must be wondering that why the number of electrons lost and gained is not the same! This is because it is only half of the story.
What happens is that two sodium atoms lose one electron each and forming two Na+ ions and releasing two electrons, and one chlorine molecule (remember, chlorine exists as a diatomic molecule) gains two electrons to form two Cl– ions. Ultimately, the number of electrons lost and gained are the same.
Oxidation Number in Covalent bonding
When a covalent bond is formed there is no loss or gain of electron(s) as an electron pair is shared to form a covalent bonding. To view the different types of covalent bonding, please view this article “Covalent Bonding“.
So you must be thinking as there is no gain or loss of electrons, then the elements or species will have an oxidation number of zero at the end.
But you would need to keep one thing in mind, different elements have different electronegativities and will attract electrons differently. The topic of electronegativity is explained in details in the following article “Electronegativity and Bond Polarity“
When a covalent bond is formed, due to the difference in electronegativity there is a shift in electron density. The more electronegative element pulls the bond pair closer towards itself. But the electrons are not fully lost or gained.
As a result, the molecule has a partial positive charge on one side and a partial negative charge on the other side. The molecule becomes a polar molecule.
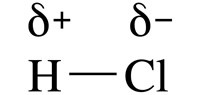
To represent this shift in electron density, the oxidation number is used. To make things easier, it is assumed that there is indeed gain or loss of electrons.
Some common elements are assigned an oxidation number whenever they do covalent bonding to form a molecule. For example, hydrogen is assigned with an oxidation number of +1, chlorine with – 1, oxygen with an oxidation number of -2. Please refer to the rules about oxidation number, covered earlier in this article.
Examples of Finding out Oxidation Numbers
1) Finding the oxidation number of Nitrogen in Ammonia (NH3)
We, know when hydrogen is combined with other elements in a covalent compound it’s oxidation number is +1, the molecule of ammonia also does not have an overall charge, hence the sum of oxidation number is zero.
So we can write:
N + (3 x +1) = 0
N + 3 = 0
N = -3
Hence, the oxidation number of Nitrogen is -3 in the ammonia molecule.
2) Finding the oxidation number of Phosphorous in the molecule Phosphorous Pentachloride (PCl5):
We know when chlorine is combined in a compound other than Fluorine, it will have an oxidation number of -1, also the molecule of phosphorous pentachloride does not have an overall charge, hence the sum of oxidation number of all the elements in the compound is 0.
So we can write:
P + (5 x -1) = 0
P -5 = 0
P = +5
Hence, the oxidation number of Phosphorous is +5 in PCl5
3) Finding the oxidation number of manganese in the manganate ion (MnO4–) :
We know, when oxygen is combined with any element other than fluorine, their oxidation number is -2, here the ion has an overall charge of -1, hence the sum of oxidation number is -1.
So we can write:
Mn + (4 x -2) = -1
Mn -8 = -1
Mn = -1+8 = +7
Hence, the oxidation number of manganese is +7 in the manganese ion.
Symbol Equation, Ionic Equation, and Half-equations:
Equations help us to understand reactions better, with Ionic and Half-equations we get a better idea of which elements are oxidized and which are reduced.
Example One: Reaction of sodium with oxygen
Symbol Equation: 4Na (s) + O2(g) → 2Na2O(s)
Ionic Equation: To break down an equation into an ionic equation, consider the following concepts, a molecule does not contain ions.
Hence the ionic equation is:
4Na (s) + O2(g) → 4Na+(s) + O2-(g)
Half-equations: Half-equations tells us which elements have gained electrons and which ones have lost. Over here we can see that 4 sodium atoms lose one electron each to form to form four Na+ ions and release four electrons in total.
First half-equation: 4Na → 4Na+ + 4e–
An oxygen molecule which consists of two oxygen atoms gains two electrons each to form two oxide (O2-) ions
Second Half-equation: O2 + 4e– → 2O2-
So by having a look at the half-equations, we can easily state that sodium is oxidized as it loses an electron and oxygen is reduced as it gains two electrons.
Example Two: Reaction of sodium with water
Symbol Equation: 2Na(s) + 2H2O(l) → 2NaOH(aq) + H2(g)
Ionic Equation: Here the H2O and H2 are molecules and do not contain any ions
Ionic Equation: 2Na+ 2H2O→ 2Na+ + 2OH– + H2
Half-Equations:
First Half-Equation: Na →Na+ + e–
Second Half-Equation: 2H2O + 2e– → 2OH– + H2
Example Three: Reaction of Magnesium with Hydrochloric acid
Symbol Equation: Mg(s) + 2HCl(aq) → MgCl2(aq) + H2(g)
Ionic Equation: Mg + 2H+ + 2Cl– → Mg2+ + 2Cl– + H2, here the chloride ion is same on both sides, its a spectator ion, hence we will exclude it from our final ionic equation.
Final Ionic Equation: Mg + 2H+ → Mg2+ + H2
Half-Equations:
First Half-Equation: Mg → Mg2+ + 2e–
Second Half-equation: 2H+ + 2e– → H2
Using Oxidation Numbers to name Compounds
We can use the concept of oxidation number to name compounds. The same element may have different oxidation numbers in different compounds, we use that concept to name certain compounds.
Examples:
- PCl3– the following compound is traditionally known as Phosphorous trichloride. In this compound phosphorous has an oxidation number of +3. If we use the concept of oxidation number to name the compound, then its name would be – Phosphorous(III)Chloride.
The roman three represents the oxidation number of phosphorous. - PCl5 -the following compound is traditionally known as phosphorous pentachloride. In this compound phosphorous has an oxidation number of +5. If we use the concept of oxidation number to name the compound, then its name would be – phosphorous (V) chloride.
- MnO4– – in this complex ion, the oxidation number of the Manganese is +7, hence the following ion is known as manganate(VII)
- CrO4– – in this complex ion chromium has an oxidation number of +6, hence the ion is known as chromate(VI)
IUPAC (International Union of Pure and Applied Chemistry) systematic names
When we name a compound using the concept of oxidation number, they are said to have IUPAC (International Union of Pure and Applied Chemistry) systematic names. But we also use traditional names for the ease of use.
The table below shows some examples of compounds/ions named with both the IUPAC naming system and the traditional names.
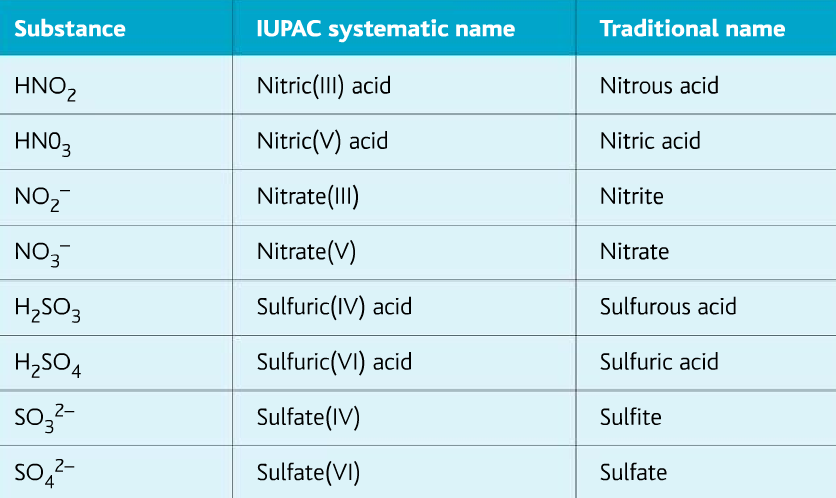
Determining whether a substance is oxidized or reduced in terms of oxidation number:
We can use the concept of oxidation number to easily determine whether a substance is oxidized or reduced. For example, after a reaction takes place and the oxidation number of a substance increases, then it is oxidized. But if a reaction occurs and the oxidation number of a substance decreases, then it is reduced.
By this time I hope you know how to calculate the oxidation number of an element in a compound as this topic has already been covered. Please view the earlier part of this article to clear idea if you are confused.
Example 1: Cl2(g) + H2S(g) → 2HCl(g) + S(s)
Before the reaction takes place, chlorine is combined to itself. It is in the form of diatomic chlorine molecule, hence the oxidation number of chlorine is 0 prior the reaction occurs.
After the reaction takes place, the chlorine has an oxidation number of -1.
The oxidation number of chlorine decreases from 0 to -1, hence chlorine is reduced.
On the other hand, the oxidation number of sulfur in H2S is -2 ( hydrogen has an oxidation number of +1 when combined in a compound), which changes to only sulfur in uncombined form with an oxidation number of 0.
The oxidation number of sulfur increases from -2 to 0, hence it is oxidized.
As both reduction and oxidation occur in the same reaction, hence this reaction is a REDOX reaction.
Test Yourself: Fe2O3 (s) + 2Al(s) → 2Fe(l) + Al2O3(s)
Explain in terms of oxidation number whether iron is oxidized or reduced
Disproportionation Reaction
What is disproportionation reaction? It is a reaction in which an element is both oxidized and reduced. Let’s have a look at an example to have a better understanding.
2H2O2 (aq) ⟶2H2O (l) + O2(g)
Oxygen had a -1 oxidation number in Hydrogen Peroxide (H2O2) but after the reaction takes place, the oxidation number of oxygen in water is -2 and 0 in gaseous oxygen.
The oxidation number of oxygen decreases from -1 to -2, meaning reduction.
The oxidation number of oxygen also increases from -1 to 0, meaning oxidation.
As the same element oxygen is both oxidized and reduced, the following reaction is a disproportionation reaction.
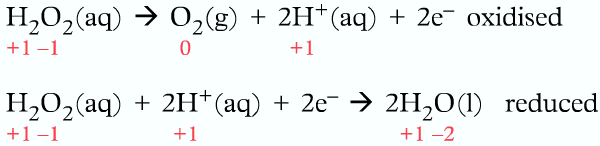
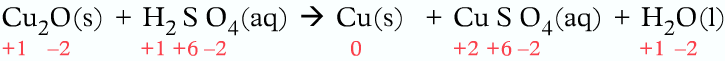
Example 2:
In the following reaction above, before the reaction copper had an oxidation number of -1. After the reaction took place, the oxidation number of copper became 0 in solid copper and +2 in copper (II) sulfate.
The oxidation number of copper decreased from +1 to 0, meaning reduction.
Also, the oxidation number of copper increased from +1 to +2, meaning oxidation.
As the same element copper is both oxidized and reduced, hence it is a disproportionation reaction.
Different Colors in different oxidation numbers
Some elements show different colors in different oxidation numbers. This is amazing because we are able to see the color change ourselves during a reaction when a change in oxidation number occurs.
Vanadium(V) ⟶ yellow
Vanadium(IV) ⟶ Blue
Vanadium(III) ⟶ Green
Vanadium(II) ⟶ Purple
Combining Half-Equations to form a balanced ionic equation
Well, this part is a bit tricky… but if you get the concept cleared up then its very easy indeed. For example, when you buy something from a shopkeeper when you give the money you lose it, but on the other hand, the shopkeeper is gaining the money. Both of you gain and lose the same amount of money.
The same case is with the electrons, the number of electrons lost should always be equal to the number of electrons gained. So when trying to balance and combining half-equations we should always balance the electron numbers first, then cancel them out. This is very similar to simultaneous equations.
Example 1
Combining the half-equations for the reaction between manganate(VII) ions in acidic solution and iron(II) ions
MnO4– (aq) + 8H+(aq) +5e– ⟶ Mn2+(aq) + 4H2O(l)
Fe2+(aq) ⟶ Fe3+(aq) + e–
Now we need to first need to multiply the second half-equation by 5, so the electron numbers become equal and they cancel each other out, just like in simultaneous equations.
MnO4– (aq) + 8H+(aq) +5e– ⟶ Mn2+(aq) + 4H2O(l)
5Fe2+(aq) ⟶ 5Fe3+(aq) + 5e–
Hence the overall ionic equation is:
MnO4– (aq) + 8H+(aq) + 5Fe2+(aq) ⟶ Mn2+(aq) + 4H2O(l) + 5Fe3+(aq)
Example 2
Combining the half-equations for the reaction between dichromate(VII) and hydrogen peroxide solution
Cr2O72-(aq) + 14H+(aq) + 6e– ⟶ 2Cr3+(aq) + 7H2O(l)
H2O2(aq) ⟶ O2(g) + 2H+(aq) + 2e–
Firstly, multiply the second equation by three to match the number of electron and then add them together.
Cr2O72-(aq) + 14H+(aq) + 6e– ⟶ 2Cr3+(aq) + 7H2O(l)
3H2O2(aq) ⟶ 3O2(g) + 6H+(aq) + 6e–
Hence the overall equation is:
Cr2O72-(aq) + 14H+(aq) + 3H2O2(aq) ⟶ 2Cr3+(aq) + 7H2O(l) + 3O2(g)
Please rembember to practice combining the half-equation part, getting it the first time is a bit tricky, but once you get it, its really easy.
If you have gone through all through this article than thanks for your patience, hope it helps you.


Top notch solid material
Its super helpful😊👍
Thank you!